(ReMoV)X2 (X = S, Se) Ternary Alloy Nanosheets for Enhanced Electrocatalytic Hydrogen Evolution Reaction
- PMID: 40270301
- PMCID: PMC12177864
- DOI: 10.1002/smll.202503399
(ReMoV)X2 (X = S, Se) Ternary Alloy Nanosheets for Enhanced Electrocatalytic Hydrogen Evolution Reaction
Abstract
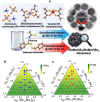
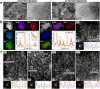
Modulating the electronic structure of 2D transition metal dichalcogenides via alloying can extend their potential applications. In this study, composition-tuned ternary alloy nanosheets of (ReMoV)X2 (X = S and Se) are synthesized using solvothermal and colloidal reactions, respectively. Ternary alloying occurred with homogeneous atomic mixing over a wide range of compositions (xV = 0.16-0.80). Compared to (ReV)X2 binary alloying, ternary alloying produces a more metallic phase with less oxidation. Increasing xV induces a phase change into a more metallic 1T phase. The (ReMoV)S2 nanosheets demonstrate enhanced electrocatalytic activity toward the acidic hydrogen evolution reaction (HER) compared to (ReV)S2. Density functional theory calculations predict that ternary alloying increases the metallicity of the nanosheets. In addition, the Gibbs free energy calculation for hydrogen adsorption (ΔGH*) shows that ternary alloying effectively activates the basal S atoms for the HER, supporting the enhanced catalytic performance observed experimentally.
Keywords: ReMoV ternary alloys; first‐principles calculations; hydrogen evolution reaction; metallic nanosheets; transition metal dichalcogenide.
© 2025 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Jaramillo T. F., Jørgensen K. P., Bonde J., Nielsen J. H., Horch S., Chorkendorff I., Science 2007, 317, 100. - PubMed
-
- Kibsgaard J., Chen Z., Reinecke B. N., Jaramillo T. F., Nat. Mater. 2012, 11, 963. - PubMed
-
- Voiry D., Yamaguchi H., Li J., Silva R., Alves D. C. B., Fujita T., Chen M., Asefa T., Shenoy V. B., Eda G., Chhowalla M., Nat. Mater. 2013, 12, 850. - PubMed
-
- Jin H., Guo C., Liu X., Liu J., Vasileff A., Jiao Y., Zheng Y., Qiao S. Z., Chem. Rev. 2018, 118, 6337. - PubMed
-
- Zhang X., Lai Z., Ma Q., Zhang H., Chem. Soc. Rev. 2018, 47, 3301. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

