Placental and Cord Blood DNA Methylation Changes Associated With Gestational Diabetes Mellitus in a Marginalized Population: The Untold Role of Saturated Fats
- PMID: 40270325
- PMCID: PMC12189174
- DOI: 10.1002/mnfr.70058
Placental and Cord Blood DNA Methylation Changes Associated With Gestational Diabetes Mellitus in a Marginalized Population: The Untold Role of Saturated Fats
Abstract
The role of DNA methylation (DNAm) and its modulation by dietary factors in gestational diabetes mellitus (GDM) remains underexplored, particularly in marginalized populations. This study investigates DNAm alterations in GDM-exposed cord blood and placenta and their association with maternal dietary quality and single nutrient intake in a low-income population from the Myanmar-Thailand border. A matched case-control design (GDM: n = 38, controls: n = 34) was selected from a Myanmar-Thailand pregnancy cohort. Dietary intake was assessed via 24-h recalls and analyzed using Nutritionist Pro, with dietary quality evaluated by the healthy eating index (HEI). DNAm was profiled in 72 cord blood and 72 placental samples using the Infinium MethylationEPIC array. Significant differences in dietary vitamin D, total folate, and saturated fat intake were observed between the groups. RnBeads analyses revealed hypomethylation as the predominant DNAm pattern in GDM, particularly at ADORA2B (placenta) and ZFP57 (cord blood) promoters. The excessive intake of saturated fats was associated with GDM hypomethylation profiles and negatively correlated with ZFP57 methylation levels. This study highlights the influence of saturated fat intake on epigenetic changes in pregnancy, revealing potential biomarkers for GDM and emphasizing the need for tailored, population-specific nutritional interventions to mitigate transgenerational health impacts.
Keywords: DNA hypomethylation; gestational diabetes mellitus; marginalized populations; maternal diet; saturated fats.
© 2025 The Author(s). Molecular Nutrition & Food Research published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
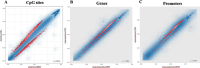
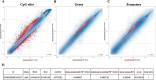
Figures






References
-
- Sweeting A., Hannah W., Backman H., et al., “Epidemiology and Management of Gestational Diabetes,” The Lancet 404, no. 10448 (2024): 175–192. - PubMed
-
- Carrasco‐Wong I., Moller A., Giachini F. R., et al., “Placental Structure in Gestational Diabetes Mellitus,” Biochimica Et Biophysica Acta (BBA)—Molecular Basis of Disease 1866, no. 2 (2020): 165535. - PubMed
-
- Quintanilla Rodriguez B. S., Vadakekut E. S., and Mahdy H., “Gestational Diabetes, in StatPearls. 2024, StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.: Treasure Island (FL) Relationships With Ineligible Companies. Disclosure: Elsa Vadakekut Declares no Relevant Financial Relationships With Ineligible Companies,” Disclosure: Heba Mahdy Declares No Relevant Financial Relationships with Ineligible Companies .
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

