Proteomic analysis of infiltrating neutrophils from rheumatoid arthritis synovial fluid and their contribution to protein carbamylation
- PMID: 40270967
- PMCID: PMC12014540
- DOI: 10.3389/fimmu.2025.1563426
Proteomic analysis of infiltrating neutrophils from rheumatoid arthritis synovial fluid and their contribution to protein carbamylation
Abstract
Introduction: Carbamylated proteins and dysregulated neutrophils are implicated in rheumatoid arthritis (RA) pathogenesis. Herein, we characterized the neutrophils present in RA synovial fluid (SF) using proteomic techniques and evaluated their contribution to protein carbamylation.
Methods: RA-SF neutrophil proteomic profile and SF proteome signature were investigated using high-resolution mass spectrometry. Carbamylated proteins and the degree of protein carbamylation were evaluated by mass spectrometric analysis. ELISA and chemiluminescence kits were used to examine myeloperoxidase (MPO) activity, and hydrogen peroxide (H2O2) generation.
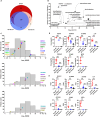
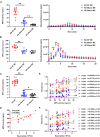
Results: SF neutrophils exhibited a shift in proteomic cargo with up-regulated proteins involved in defense responses, neutrophil degranulation, and reactive oxygen species metabolic processes, while proteins down-regulated were associated with megakaryocyte differentiation, leukocyte migration, and integrin-mediated signaling pathway. Elevated levels of neutrophil-derived proteins were detected in RA-SF. In addition, we specifically identified many carbamylated proteins and observed an increased frequency of protein carbamylation in RA-SF samples. Functionally, neutrophils from RA-SF showed a significantly increased level of MPO release and HH2O2 generation. Moreover, MPO activity was higher in RA-SF than in autologous blood samples, which correlated well with the degree of protein carbamylation in RA-SF.
Discussion: Synovial neutrophils were found to be activated and increased releasing protein cargo, including MPO and ROS, into the synovial fluid. Presence of many carbamylated proteins in RA-SF and an increased MPO activity showed a strong correlation to the degree of protein carbamylation, suggesting neutrophil-derived MPO in promoting generation of aberrantly carbamylated proteins.
Keywords: carbamylation; myeloperoxidase; neutrophil; rheumatoid arthritis; synovial fluid.
Copyright © 2025 Chen, Du, Wang, Qiu, Chen, Wan, Qiu, Xiong, Nandakumar, Holmdahl and Geng.
Conflict of interest statement
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Rheumatoid Arthritis Synovial Fluid Neutrophils Drive Inflammation Through Production of Chemokines, Reactive Oxygen Species, and Neutrophil Extracellular Traps.Front Immunol. 2021 Jan 5;11:584116. doi: 10.3389/fimmu.2020.584116. eCollection 2020. Front Immunol. 2021. PMID: 33469455 Free PMC article.
-
Activated neutrophil carbamylates albumin via the release of myeloperoxidase and reactive oxygen species regardless of NETosis.Mod Rheumatol. 2020 Mar;30(2):345-349. doi: 10.1080/14397595.2019.1583819. Epub 2019 May 2. Mod Rheumatol. 2020. PMID: 30789095
-
Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility.Arthritis Res Ther. 2014 Jun 13;16(3):R122. doi: 10.1186/ar4579. Arthritis Res Ther. 2014. PMID: 24928093 Free PMC article.
-
Redox-Mediated Carbamylation As a Hapten Model Applied to the Origin of Antibodies to Modified Proteins in Rheumatoid Arthritis.Antioxid Redox Signal. 2022 Mar;36(7-9):389-409. doi: 10.1089/ars.2021.0064. Epub 2021 Jun 4. Antioxid Redox Signal. 2022. PMID: 33906423 Free PMC article. Review.
-
Proteomic analysis of synovial fluid: current and potential uses to improve clinical outcomes.Expert Rev Proteomics. 2019 Apr;16(4):287-302. doi: 10.1080/14789450.2019.1578214. Epub 2019 Feb 22. Expert Rev Proteomics. 2019. PMID: 30793992 Review.
Cited by
-
The Role and Mechanism of Protein Post‑Translational Modification in Rheumatoid Arthritis.J Inflamm Res. 2025 Jul 11;18:9055-9078. doi: 10.2147/JIR.S528487. eCollection 2025. J Inflamm Res. 2025. PMID: 40666378 Free PMC article. Review.
References
-
- Shi J, Knevel R, Suwannalai P, van der Linden MP, Janssen GM, van Veelen PA, et al. . Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci United States America. (2011) 108:17372–7. doi: 10.1073/pnas.1114465108 - DOI - PMC - PubMed
-
- Castellanos-Moreira R, Rodríguez-García SC, Gomara MJ, Ruiz-Esquide V, Cuervo A, Casafont-Solé I, et al. . Anti-carbamylated proteins antibody repertoire in rheumatoid arthritis: evidence of a new autoantibody linked to interstitial lung disease. Ann rheumatic Dis. (2020) 79:587–94. doi: 10.1136/annrheumdis-2019-216709 - DOI - PubMed
-
- Humphreys JH, Verheul MK, Barton A, MacGregor AJ, Lunt M, Toes RE, et al. . Anticarbamylated protein antibodies are associated with long-term disability and increased disease activity in patients with early inflammatory arthritis: results from the Norfolk Arthritis Register. Ann rheumatic Dis. (2016) 75:1139–44. doi: 10.1136/annrheumdis-2015-207326 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

