Early experience and assessment of real-world toxicities with mirvetuximab soravtansine in a heavily pretreated patient cohort with ovarian cancer
- PMID: 40270982
- PMCID: PMC12013391
- DOI: 10.1016/j.gore.2025.101738
Early experience and assessment of real-world toxicities with mirvetuximab soravtansine in a heavily pretreated patient cohort with ovarian cancer
Abstract
Introduction: Mirvetuximab soravtansine (MIRV) recently emerged as a promising therapeutic option for patients with platinum resistant ovarian cancer (PROC). In trials leading up to its approval, several ocular and other toxicities were identified. We report our experience with MIRV in a less-selected "real-world" population.
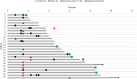
Methods: A retrospective review of patients with folate-receptor alpha positive, recurrent PROC treated with MIRV between December 2022-April 2024 was performed. Demographic, treatment, and toxicity data were abstracted from the medical record. The primary outcomes of interest were the incidence and nature of MIRV-related toxicities, as well as the clinical response to treatment.
Results: 25 patients were analyzed, and 44 % had >3 previous lines of treatment. The median duration of MIRV treatment 4.7 months, and 36 % of patients received more than 6 months of MIRV. Ocular events were common and occurred early, resulting in grade 1, 2, and 3 toxicity (28 %, 20 %, and 16 % respectively). As a result, 36 % of patients had an increased frequency of ophthalmologic care, 48 % required a medication change, and 32 % had dose reduction, but none required MIRV discontinuation. Pneumonitis occurred in 24 % of patients, was the leading cause of treatment discontinuation and may be associated with significant underlying cardiopulmonary comorbidities and pulmonary disease burden.
Conclusion: Our real-world experience with MIRV mirrors previously published data and suggests a benefit may also be seen in a more heavily pretreated population. Toxicities were similar to those previously reported, although our increased incidence of pneumonitis suggests care when treating patients with significant underlying cardiopulmonary comorbidities.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Coleman R.L., Lorusso D., Oaknin A., Cecere S.C., Denys H., Colombo N., et al. Mirvetuximab soravtansine in folate receptor alpha (FRα)–high platinum-resistant ovarian cancer: final overall survival and post hoc sequence of therapy subgroup results from the SORAYA trial. Int. J. Gynecol. Cancer. 2024;34(8):1119–1125. - PMC - PubMed
-
- Common Terminology Criteria for Adverse Events (CTCAE). 2017.
-
- Gyawali B., Parsad S., Feinberg B.A., Nabhan C. Real-world evidence and randomized studies in the precision oncology era: the right balance. JCO Precis. Oncol. 2017;1:1–5. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous