Synthesis and reactivity of a parent phosphathioethynolato-borane and a boraarsaketene
- PMID: 40271024
- PMCID: PMC12012630
- DOI: 10.1039/d5sc01996f
Synthesis and reactivity of a parent phosphathioethynolato-borane and a boraarsaketene
Abstract
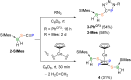
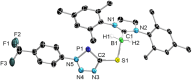
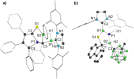
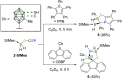
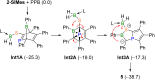
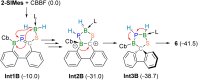
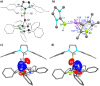
We present the synthesis and isolation of the first main-group phosphathioethynolates, [LBH2(SCP)] (L = SIMes, CAACMe; SIMes = 1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidene, CAACMe = 1-(2,6-diisopropylphenyl)-3,3,5,5-tetramethylpyrrolidin-2-ylidene), in which phosphathioethynolate coordination occurs exclusively through the sulfur atom, giving rise to a phosphaalkyne-type structural motif. This is reflected in the reactivity of the SIMes derivative towards organic azides and [(η2-C2H4)2CoCp] (Cp = C5H5), which mirrors the behavior of 1-adamantylphosphaalkyne, yielding triazaphospholes and a mixed cyclopentadienyl-(1,3-diphosphete) sandwich complex, respectively. Deviations from typical phosphaalkyne reactivity are observed in reactions with boron-containing heterocycles such as pentaphenylborole (PPB) and a carboranyl-substituted 9-borafluorene, which yield an unprecedented bicyclic structure and a zwitterionic spiro compound, respectively. Furthermore, we report the synthesis of the first arsaketenylborane, [(SIMes)BH2(AsCO)], which, although too unstable for isolation, generates an arsinidene in situ, which can be trapped by coordination to a PPB C[double bond, length as m-dash]C double bond.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Weber L. Eur. J. Inorg. Chem. 2018;2018:2175–2227. doi: 10.1002/ejic.201800179. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

