Interpreting the clinical significance of multiple large-scale mitochondrial DNA deletions (MLSMD) in skeletal muscle tissue in the diagnostic evaluation of primary mitochondrial disease
- PMID: 40271067
- PMCID: PMC12015102
- DOI: 10.3389/fphar.2025.1507493
Interpreting the clinical significance of multiple large-scale mitochondrial DNA deletions (MLSMD) in skeletal muscle tissue in the diagnostic evaluation of primary mitochondrial disease
Abstract
Background and objectives: Improved detection sensitivity from combined Long-Range PCR (LR-PCR), Next-Generation Sequencing (NGS), and droplet digital PCR (ddPCR) to identify multiple large-scale mtDNA deletions (MLSMD) and quantify deletion heteroplasmy have introduced clinical interpretation challenges. We sought to evaluate clinical, biochemical, and histopathological phenotypes of a large clinical cohort harboring MLSMD in muscle to better understand their significance across a range of clinical phenotypes.
Methods: A single-site retrospective study was performed of 212 diagnostic muscle biopsies obtained from patients referred for Primary Mitochondrial Disease (PMD) evaluation with muscle mitochondrial (mt)DNA sequencing performed at our institution, including electronic medical record (EMR) review of symptoms, biochemical results, and Mitochondrial Myopathy Composite Assessment Tool (MM-COAST) scores.
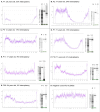
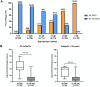
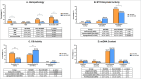
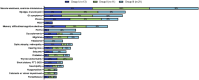
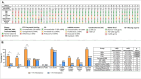
Results: MLSMD were identified in 50 of 212 (24%) diagnostic tissue biopsies, and were universally present. in subjects ≥50 years (n = 18/18). In 45 of 50 (90%) subjects with MLSMD, no definitive genetic etiology was identified, despite clinical whole exome sequencing (WES) and/or whole genome sequencing (WGS). MLSMD heteroplasmy levels quantified by ddPCR ranged from 0% to 33%, exceeding 10% heteroplasmy in 5/45 (11%). Subjects with MLSMD (n = 45) were more likely to demonstrate mitochondrial abnormalities on histopathology, upregulation (≥150% of control mean) of one or more electron transport chain (ETC) complex enzyme activities, and reduced citrate synthase indicative of mitochondrial depletion (<60% of control mean) relative to subjects without MLSMD (n = 155). As clinical phenotypes varied across the MLSMD cohort, Bernier diagnostic criteria major/minor symptoms were used to discriminate 13 of 45 subjects with "suspected" PMD having unrevealing WES/WGS results and 32 of 45 subjects scored as "less likely" to have PMD. Relative to the "less likely" cohort, a significantly higher frequency of biochemical and muscle histopathological abnormalities (ragged red and COX negative fibers) were observed in the "suspected" cohort, further supporting a higher index of suspicion for PMD, p < 0.05.
Discussion: MLSMD in skeletal muscle tissue were a common molecular finding (24%) in our cohort and consistently present in subjects ≥50 years. Among those with genetically undiagnosed MLSMD (n = 45), the "suspected" PMD subset (n = 13/45) represent a promising cohort for novel gene discoveries.
Keywords: electron transport chain (ETC) enzymatic activity; mitochondrial DNA (mtDNA); multiple large-scale mitochondrial DNA deletions (MLSMD); primary mitochondrial disease (PMD); ragged blue fibers (RBF); ragged red fibers (RRF).
Copyright © 2025 Wang, Peterson, Santos, Chan, Diaz-Miranda, Rahaman, Flickinger, Goldstein, Bogush, McCormick, Muraresku, Anderson, Dulik, Wallace, Xiao, Falk, Viaene and Zolkipli-Cunningham.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bychkov I. O., Itkis Y. S., Tsygankova P. G., Krylova T. D., Mikhaylova S. V., Klyushnikov S. A., et al. (2021). Mitochondrial DNA maintenance disorders in 102 patients from different parts of Russia: Mutational spectrum and phenotypes. Mitochondrion 57, 205–212. 10.1016/j.mito.2021.01.004 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

