Citrullination in health and disease: From physiological function to gene regulation
- PMID: 40271192
- PMCID: PMC12017988
- DOI: 10.1016/j.gendis.2024.101355
Citrullination in health and disease: From physiological function to gene regulation
Abstract
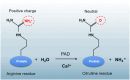
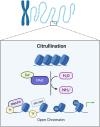
Protein citrullination involves the deimination of arginine or methylarginine residues in peptide chains to form citrulline by peptidyl arginine deiminases. This process is an important protein post-translational modification that affects molecular structure and function of various proteins, including histones. In recent years, protein citrullination has attracted widespread attention for its influence on gene transcription. Studies on the impact of protein citrullination modification on chromatin structure remodeling and the establishment of gene regulatory networks have made rapid progress. In this review, we briefly summarize the physiological functions of protein citrullination modification. Specifically, we comprehensively outline the latest progress in the study of the role of protein citrullination modification in gene transcription regulation, focusing on the interaction of protein citrullination with other post-translational modifications.
Keywords: Citrullination; Deimination; Histone; Peptidyl arginine deiminase; Therapeutic interventions; Transcriptional control.
© 2024 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co., Ltdé.
Figures

References
-
- Horibata S.C.S., Cherrington B.D. Role for peptidylarginine deiminase enzymes in disease and female reproduction. J Reprod Dev. 2012;58(3):274–282. - PubMed
-
- Arita K.H.H., Shimizu T., Nakashima K., Yamada M., Sato M. Structural basis for Ca(2+)-induced activation of human PAD4. Nat Struct Mol Biol. 2004;11(8):777–783. - PubMed
-
- Cosgrove M.S., Wolberger C. How does the histone code work? Biochem Cell Biol. 2005;83(4):468–476. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

