Single step site-selective reaction to construct a Ag2Au2 ← Ag4 supramolecular assembly from hybrid N-heterocyclic carbene (NHC): synthesis, structures and optoelectronic properties
- PMID: 40271410
- PMCID: PMC12017385
- DOI: 10.1039/d5ra00684h
Single step site-selective reaction to construct a Ag2Au2 ← Ag4 supramolecular assembly from hybrid N-heterocyclic carbene (NHC): synthesis, structures and optoelectronic properties
Abstract
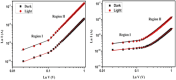
Two supramolecular complex assemblies, [Ag4(1)2][PF6]4·4MeCN 2 and Ag(i)-Au(i) mixed metal complex [Ag2Au2(1)2][PF6]4·4MeCN 3, have been prepared from 3-(pyridylmethyl)imidazo[1,5-a]pyridin-4-ylium hexafluorophosphate (1 HPF6), which is the precursor of N-heterocyclic carbene (NHC). These complexes were subsequently analyzed using various spectroscopic techniques to confirm their structural and chemical properties. Transmetallation of Au(i) onto the Ag4 macrocycle results in the formation of an Ag2Au2 macrocyclic assembly. Au(i) selectively binds with the soft donor Ccarbene, whereas Ag(i) binds with comparatively hard donor Npy (py = pyridine). The geometries of 2 and 3 were established by single-crystal X-ray diffraction studies. Both molecules form a 2D network through M-M and several non-covalent interactions. Electrical conductivity measurements revealed that Ag(i) complex 2 is better conductor than Au(i) complex 3. Optoelectronic studies revealed the utility of complexes 2 and 3 as photovoltaic devices. Furthermore, MS-junction potential measurements show that they are suitable for semiconductor devices, with complex 2 being more efficient than complex 3. Finally, in this study, we aimed to explore the scope of (i) the development of heterobimetallic supramolecular organometallic complexes (SOC), (ii) the charge transport behaviour of SOCs, and (iii) the modification of intrinsically conductive SOCs-based electronics.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures






Similar articles
-
Metallosupramolecular Architectures Obtained from Poly-N-heterocyclic Carbene Ligands.Acc Chem Res. 2017 Sep 19;50(9):2167-2184. doi: 10.1021/acs.accounts.7b00158. Epub 2017 Aug 25. Acc Chem Res. 2017. PMID: 28841284
-
Unexpected structural preference with metallophilic Ag-Au contacts in silver(I)-N-heterocyclic carbene cluster; experimental and theoretical approach.Dalton Trans. 2024 Jan 16;53(3):1099-1104. doi: 10.1039/d3dt02551a. Dalton Trans. 2024. PMID: 38099652
-
Strategy for the Construction of Diverse Poly-NHC-Derived Assemblies and Their Photoinduced Transformations.Angew Chem Int Ed Engl. 2020 Jun 15;59(25):10073-10080. doi: 10.1002/anie.201912322. Epub 2019 Oct 28. Angew Chem Int Ed Engl. 2020. PMID: 31589799
-
Heterobimetallic N-Heterocyclic Carbene Complexes: A Synthetic, Spectroscopic, and Theoretical Study.Inorg Chem. 2016 Jul 18;55(14):6882-91. doi: 10.1021/acs.inorgchem.6b00222. Epub 2016 Mar 26. Inorg Chem. 2016. PMID: 27018229
-
Synthesis, characterization, and structure-activity relationship of the antimicrobial activities of dinuclear N-heterocyclic carbene (NHC)-silver(I) complexes.J Inorg Biochem. 2016 Oct;163:110-117. doi: 10.1016/j.jinorgbio.2016.06.031. Epub 2016 Jun 29. J Inorg Biochem. 2016. PMID: 27426050
References
-
- Chakrabarty R. Mukherjee P. S. Stang P. J. Chem. Rev. 2011;111:6810–6918. - PMC - PubMed
- Goswami A. Saha S. Biswas P. K. Schmittel M. Chem. Rev. 2020;120:125–199. - PubMed
- Fujita M. Tominaga M. Hori A. Therrien B. Acc. Chem. Res. 2005;38:369–378. - PubMed
- Gao W.-X. Feng H.-J. Guo B.-B. Lu Y. Jin G.-X. Chem. Rev. 2020;120:6288–6325. - PubMed
- Yoshizawa M. Catti L. Acc. Chem. Res. 2019;52:2392–2404. - PubMed
- Fujita D. Ueda Y. Sato S. Mizuno N. Kumasaka T. Fujita M. Nature. 2016;540:563–566. - PubMed
- Hiraoka S. Harano K. Shiro M. Ozawa Y. Yasuda N. Toriumi K. Shionoya M. Angew. Chem., Int. Ed. 2006;45:6488–6491. - PubMed
- Wang S.-C. Cheng K.-Y. Fu J.-H. Cheng Y.-C. Chan Y.-T. J. Am. Chem. Soc. 2020;142:16661–16667. - PubMed
- Cai L.-X. Yan D.-N. Cheng P.-M. Xuan J.-J. Li S.-C. Zhou L.-P. Tian C.-B. Sun Q.-F. J. Am. Chem. Soc. 2021;143:2016–2024. - PubMed
-
- Meeuwissen J. Reek J. N. Nat. Chem. 2010;2:615–621. - PubMed
- De S. Mahata K. Schmittel M. Chem. Soc. Rev. 2010;39:1555–1575. - PubMed
- Li J.-R. Zhou H.-C. Nat. Chem. 2010;2:893–898. - PubMed
- Yamanaka M. Yamada Y. Sei Y. Yamaguchi K. Kobayashi K. J. Am. Chem. Soc. 2006;128:1531–1539. - PubMed
- Sun Q.-F. Sato S. Fujita M. Angew. Chem., Int. Ed. 2014;53:13510–13513. - PubMed
- Ayme J.-F. Beves J. E. Campbell C. J. Leigh D. A. J. Am. Chem. Soc. 2019;141:3605–3612. - PMC - PubMed
- Li G. Zhou Z. Yuan C. Guo Z. Liu Y. Zhao D. Liu K. Zhao J. Tan H. Yan X. Angew. Chem., Int. Ed. 2020;59:10013–10017. - PubMed
-
- Mal P. Breiner B. Rissanen K. Nitschke J. R. Science. 2009;324:1697–1699. - PubMed
- Rizzuto F. J. von Krbek L. K. S. Nitschke J. R. Nat. Chem. Rev. 2019;3:204–222.
- Niki K. Tsutsui T. Yamashina M. Akita M. Yoshizawa M. Angew. Chem., Int. Ed. 2020;59:10489–10492. - PubMed
- Howlader P. Mondal B. Purba P. C. Zangrando E. Mukherjee P. S. J. Am. Chem. Soc. 2018;140:7952–7960. - PubMed
- Wang L.-J. Li X. Bai S. Wang Y.-Y. Han Y.-F. J. Am. Chem. Soc. 2020;142:2524–2531. - PubMed
- Xu L. Zhang D. Ronson T. K. Nitschke J. R. Angew. Chem., Int. Ed. 2020;59:7435–7438. - PMC - PubMed
- Kieffer M. Bilbeisi R. A. Thoburn J. D. Clegg J. K. Nitschke J. R. Angew. Chem., Int. Ed. 2020;59:11369–11373. - PMC - PubMed
-
- Zhang M. Saha M. L. Wang M. Zhou Z. Song B. Lu C. Yan X. Li X. Huang F. Yin S. Stang P. J. J. Am. Chem. Soc. 2017;139:5067–5074. - PubMed
- McConnell A. J. Wood C. S. Neelakandan P. P. Nitschke J. R. Chem. Rev. 2015;115:7729–7793. - PubMed
- Saha M. L. Yan X. Stang P. J. Acc. Chem. Res. 2016;49:2527–2539. - PubMed
- Yan X. Wang H. Hauke C. E. Cook T. R. Wang M. Saha M. L. Zhou Z. Zhang M. Li X. Huang F. Stang P. J. J. Am. Chem. Soc. 2015;137:15276–15286. - PubMed
-
- Yoshizawa M. Tamura M. Fujita M. Science. 2006;312:251–254. - PubMed
- Kaphan D. M. Levin M. D. Bergman R. G. Raymond K. N. Toste F. D. Science. 2015;350:1235–1238. - PubMed
- Brown C. J. Toste F. D. Bergman R. G. Raymond K. N. Chem. Rev. 2015;115:3012–3035. - PubMed
- Jongkind L. J. Caumes X. Hartendorp A. P. T. Reek J. N. H. Acc. Chem. Res. 2018;51:2115–2128. - PMC - PubMed
- Fang Y. Powell J. A. Li E. Wang Q. Perry Z. Kirchon A. Yang X. Xiao Z. Zhu C. Zhang L. Huang F. Zhou H.-C. Chem. Soc. Rev. 2019;48:4707–4730. - PubMed
- Martí-Centelles V. Lawrence A. L. Lusby P. J. J. Am. Chem. Soc. 2018;140:2862–2868. - PubMed
- Wang Q.-Q. Gonell S. Leenders S. H. A. M. Dürr M. IvanovićBurmazović I. Reek J. N. H. Nat. Chem. 2016;8:225–230. - PubMed
- Guo J. Fan Y.-Z. Lu Y.-L. Zheng S.-P. Su C.-Y. Angew. Chem., Int. Ed. 2020;59:8661–8669. - PubMed
- Tan C. Jiao J. Li Z. Liu Y. Han X. Cui Y. Angew. Chem., Int. Ed. 2018;57:2085–2090. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

