Multiaction Antimicrobial, Anti-inflammatory, and Prohealing Hydrogel as a Novel Strategy for Preventing Postoperative Pancreatic Fistula
- PMID: 40271421
- PMCID: PMC12015097
- DOI: 10.34133/bmr.0194
Multiaction Antimicrobial, Anti-inflammatory, and Prohealing Hydrogel as a Novel Strategy for Preventing Postoperative Pancreatic Fistula
Abstract
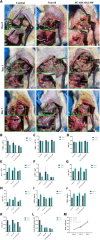
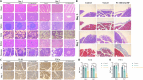
Postoperative pancreatic fistula remains a challenging complication after pancreaticoduodenectomy. Addressing this issue requires effective strategies to promote anastomotic healing. In this study, we developed a novel hydrogel designed to close pancreaticoenteric anastomosis after pancreaticoduodenectomy. The hydrogel-composed of polyvinyl alcohol, chitosan, and dopamine-modified oxidized hyaluronic acid-exhibited excellent antibacterial, anti-inflammatory, and wound healing properties. It was designed to conform well to the anastomotic site for clinical application. The hydrogel demonstrated good biocompatibility, appropriate mechanical strength, low swelling, and strong adhesive properties, meeting specific requirements for pancreaticoenteric anastomosis environments. Moreover, by activating the cell cycle, it promoted cell proliferation and migration, thereby accelerating anastomotic closure. Addition of the potent broad-spectrum antibiotic meropenem further enhanced its antibacterial efficacy, targeting common microbial species involved in delayed healing and fistula formation after pancreatic surgery. In a rat model of pancreatic fistula, the hydrogel effectively sealed the anastomosis, filled potential suture gaps, and exerted antibacterial, anti-inflammatory, and tissue regeneration-promoting effects around the anastomotic site. Therefore, this hydrogel, with its ideal degradation properties, shows promising application prospects in closing pancreaticoenteric anastomosis following pancreaticoduodenectomy, thereby offering an effective solution to reduce complications such as pancreatic fistula after pancreatic surgery.
Copyright © 2025 Yuan Zhou et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures



References
-
- Simon R. Complications after pancreaticoduodenectomy. Surg Clin North Am. 2021;101(5):865–874. - PubMed
-
- Malgras B, Dokmak S, Aussilhou B, Pocard M, Sauvanet A. Management of postoperative pancreatic fistula after pancreaticoduodenectomy. J Visc Surg. 2023;160(1):39–51. - PubMed
-
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M, et al. . International study group on pancreatic fistula, postoperative pancreatic fistula: An international study group (ISGPF) definition. Surgery. 2005;138(1):8–13. - PubMed
LinkOut - more resources
Full Text Sources

