Quality by design-based optimization and HP-TLC densitometric standardization of Theobroma cacao L. extract as a nutraceutical supplement
- PMID: 40271427
- PMCID: PMC12016216
- DOI: 10.3389/fnut.2025.1537963
Quality by design-based optimization and HP-TLC densitometric standardization of Theobroma cacao L. extract as a nutraceutical supplement
Abstract
Background: Our previous studies identified the hydroalcoholic extract of defatted Theobroma cacao L. bean (CE) as a cancer-preventive and a protective agent against chemotherapeutic-induced toxicities, specifically doxorubicin-induced heart, liver, and kidney toxicities.
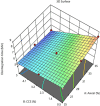
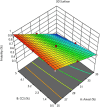
Methods: An analytical method for phytochemical standardization was developed, and acute oral toxicity was studied in female Wistar rats following the OECD 423 guidelines. In brief, the CE was extracted using an 80:20 alcohol-water (% v/v) mixture through cold maceration, followed by spray drying to obtain powdered CE. Utilizing a Quality by Design (QbD) approach with Design Expert (DoE) software, we optimized CE tablets via direct compression. The central composite design (CCD) included five center points, with Avicel PH - 101 and croscarmellose sodium (CCS) as factors, and disintegration time, hardness, and % loss due to friability as measurements.
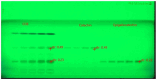
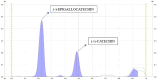
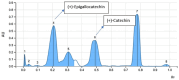
Results: Among the 13 formulations, batch F-9 emerged as the optimized one within the design space, containing 35% Avicel PH - 101 and 5% CCS. The optimized formulation exhibited a disintegration time of 5.2 min, hardness of 4.2 kg/cm2, and friability of 0.34%. Importantly, no toxic effects were found at 2,000 mg/kg in the acute oral toxicity study. CE contains vital bioactive polyphenols, including (-)-epigallocatechin-3-gallate (EGCG) and (+)-catechin (CTN). We developed a marker-based HP-TLC densitometric analysis using a mobile phase of 9:9:2 v/v [ethyl acetate: toluene: formic acid], which revealed CTN at Rf 0.49 and EGCG at Rf 0.23. This method was validated according to ICH requirements.
Conclusion: In conclusion, the novel, validated HP-TLC method simultaneously detects EGCG and CTN in the cocoa extract. Tablets formulated by direct compression are safe as nutraceuticals and hold promise as supplements in palliative cancer therapy.
Keywords: EGCG (−)-epigallocatechin-3-gallate; HPTLC; Theobroma cacao L; central composite design; nutraceuticals; quality by design.
Copyright © 2025 Muppayyanamath, Harish, Mastiholimath, Patil, Patil, Hegde and Roy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Quality-by-design based development of fast dispersible nimodipine tablets: Formulation attributes and release kinetic assessment.Pak J Pharm Sci. 2023 Mar;36(2):547-556. Pak J Pharm Sci. 2023. PMID: 37530164
-
Effect of Theobroma cacao L. on the Efficacy and Toxicity of Doxorubicin in Mice Bearing Ehrlich Ascites Carcinoma.Antioxidants (Basel). 2022 May 31;11(6):1094. doi: 10.3390/antiox11061094. Antioxidants (Basel). 2022. PMID: 35739991 Free PMC article.
-
Densitometric Quantification and Optimization of Polyphenols in Phyllanthus maderaspatensis by HPTLC.Saudi J Biol Sci. 2022 Mar;29(3):1521-1529. doi: 10.1016/j.sjbs.2021.11.019. Epub 2021 Nov 22. Saudi J Biol Sci. 2022. PMID: 35280567 Free PMC article.
-
Extraction, Identification, and Quantification of Polyphenols from the Theobroma cacao L. Fruit: Yield vs. Environmental Friendliness.Foods. 2024 Jul 29;13(15):2397. doi: 10.3390/foods13152397. Foods. 2024. PMID: 39123588 Free PMC article. Review.
-
Therapeutic potential of green tea catechin, (-)-epigallocatechin-3-O-gallate (EGCG) in SARS-CoV-2 infection: Major interactions with host/virus proteases.Phytomed Plus. 2023 Feb;3(1):100402. doi: 10.1016/j.phyplu.2022.100402. Epub 2022 Dec 30. Phytomed Plus. 2023. PMID: 36597465 Free PMC article. Review.
References
-
- Chaudhary D, Lamichhane G. Overview of natural products in nutraceutical industries In: Herbs, spices and their roles in nutraceuticals and functional foods. Amsterdam, Netherlands: Academic Press (Elsevier) (2023). 1–13.
-
- Ghumare AR, Borade VS, Talele SG, Shahare S, Pawar A, Shinde C, et al. . Formulation and evaluation of nutraceutical tablet of Polyherbal drugs by wet granulation. J Pharm Negat Resul. (2023) 14:359–368. doi: 10.47750/pnr.2023.14.02.47 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

