Thiamine, gastrointestinal beriberi and acetylcholine signaling
- PMID: 40271433
- PMCID: PMC12014454
- DOI: 10.3389/fnut.2025.1541054
Thiamine, gastrointestinal beriberi and acetylcholine signaling
Abstract
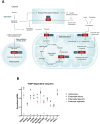
Research has highlighted numerous detrimental consequences of thiamine deficiency on digestive function. These range from impaired gastric and intestinal motility to aberrant changes in pancreatic exocrine function, gastric acidity and disturbances in gut barrier integrity and inflammation. Thiamine and its pharmacological forms, as a primary or adjunctive therapy, have been shown to improve symptoms such as nausea, constipation, dysphagia and intestinal dysmotility, in both humans and animals. This review aims to explore molecular mechanisms underlying the therapeutic action of thiamine in gastrointestinal dysfunction. Our analysis demonstrates that thiamine insufficiency restricted to the gastrointestinal system, i.e., lacking well-known symptoms of dry and wet beriberi, may arise through (i) a disbalance between the nutrient influx and efflux in the gastrointestinal system due to increased demands of thiamine by the organism; (ii) direct exposure of the gastrointestinal system to oral drugs and gut microbiome, targeting thiamine-dependent metabolism in the gastrointestinal system in the first line; (iii) the involvement of thiamine in acetylcholine (ACh) signaling and cholinergic activity in the enteric nervous system and non-neuronal cells of the gut and pancreas, employing both the coenzyme and non-coenzyme actions of thiamine. The coenzyme action relies on the requirement of the thiamine coenzyme form - thiamine diphosphate - for the production of energy and acetylcholine (ACh). The non-coenzyme action involves participation of thiamine and/or derivatives, including thiamine triphosphate, in the regulation of ACh synaptic function, consistent with the early data on thiamine as a co-mediator of ACh in neuromuscular synapses, and in allosteric action on metabolic enzymes. By examining the available evidence with a focus on the gastrointestinal system, we deepen the understanding of thiamine's contribution to overall gastrointestinal health, highlighting important implications of thiamine-dependent mechanisms in functional gastrointestinal disorders.
Keywords: acetylcholine; functional gastrointestinal disorder; gastrointestinal beriberi; intestinal ThDP-dependent enzymes; intestinal metabolism of thiamine; intestinal transport of thiamine; thiamine.
Copyright © 2025 Overton, Emelyanova and Bunik.
Conflict of interest statement
EO is an owner of a nutraceutical company that sells vitamin supplements including thiamine. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
-
- Rome IV. Criteria. Rome Found Available at: https://theromefoundation.org/rome-iv/rome-iv-criteria/ (Accessed October 31, 2024).
Publication types
LinkOut - more resources
Full Text Sources

