A host enzyme reduces metabolic dysfunction-associated steatotic liver disease (MASLD) by inactivating intestinal lipopolysaccharide
- PMID: 40271687
- PMCID: PMC12021412
- DOI: 10.7554/eLife.100731
A host enzyme reduces metabolic dysfunction-associated steatotic liver disease (MASLD) by inactivating intestinal lipopolysaccharide
Abstract
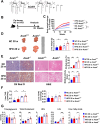
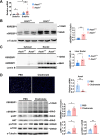
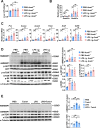
The incidence of metabolic dysfunction-associated steatotic liver disease (MASLD) has been increasing worldwide. Since gut-derived bacterial lipopolysaccharides (LPS) can travel via the portal vein to the liver and play an important role in producing hepatic pathology, it seemed possible that (1) LPS stimulates hepatic cells to accumulate lipid, and (2) inactivating LPS can be preventive. Acyloxyacyl hydrolase (AOAH), the eukaryotic lipase that inactivates LPS and oxidized phospholipids, is produced in the intestine, liver, and other organs. We fed mice either normal chow or a high-fat diet for 28 weeks and found that Aoah-/- mice accumulated more hepatic lipid than did Aoah+/+ mice. In young mice, before increased hepatic fat accumulation was observed, Aoah-/- mouse livers increased their abundance of sterol regulatory element-binding protein 1, and the expression of its target genes that promote fatty acid synthesis. Aoah-/- mice also increased hepatic expression of Cd36 and Fabp3, which mediate fatty acid uptake, and decreased expression of fatty acid-oxidation-related genes Acot2 and Ppara. Our results provide evidence that increasing AOAH abundance in the gut, bloodstream, and/or liver may be an effective strategy for preventing or treating MASLD.
Keywords: acyloxyacyl hydrolase; gut–liver axis; human; immunology; inflammation; lipopolysaccharide; metabolic dysfunction-associated steatotic liver disease; mouse; sterol regulatory element-binding protein 1.
Conflict of interest statement
ZW, NO, YL, LG, ZX, JF, WJ, YC, CY, CL, AA, XL, MF, JW, YC, RM, ML No competing interests declared, BZ affiliated with BeiGene (Shanghai) Research & Development Co., Ltd
Figures







Update of
- doi: 10.1101/2024.06.23.600304
- doi: 10.7554/eLife.100731.1
- doi: 10.7554/eLife.100731.2
References
-
- An L, Wirth U, Koch D, Schirren M, Drefs M, Koliogiannis D, Nieß H, Andrassy J, Guba M, Bazhin AV, Werner J, Kühn F. The role of gut-derived lipopolysaccharides and the intestinal barrier in fatty liver diseases. Journal of Gastrointestinal Surgery. 2022;26:671–683. doi: 10.1007/s11605-021-05188-7. - DOI - PMC - PubMed
-
- Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J, Nieuwdorp M, Clément K. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nature Reviews. Gastroenterology & Hepatology. 2020a;17:279–297. doi: 10.1038/s41575-020-0269-9. - DOI - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
- 32300772/National Natural Science Foundation of China
- 32170929/National Natural Science Foundation of China
- 32370979/National Natural Science Foundation of China
- 32260189/National Natural Science Foundation of China
- gzwkj2023-568/Science and Technology Program of Guizhou Province
- 82301980/National Natural Science Foundation of China
- AI18188/NH/NIH HHS/United States
- ZK2024 - 240/Guizhou Provincial Science and Technology Department
- AI44642/NH/NIH HHS/United States
- R56 AI018188/AI/NIAID NIH HHS/United States
- 31770993/National Natural Science Foundation of China
- R37 AI018188/AI/NIAID NIH HHS/United States
- 31570910/National Natural Science Foundation of China
- 91742104/National Natural Science Foundation of China
- 21ZR1405400/Shanghai Committee of Science and Technology
- R01 AI018188/AI/NIAID NIH HHS/United States
- P01 AI044642/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

