The Effectiveness and Safety Profile of Nivolumab-Plus-Ipilimumab in Previously Untreated Japanese Patients With Advanced or Metastatic Renal Cell Carcinoma (J-ENCORE Study)
- PMID: 40271863
- PMCID: PMC12324984
- DOI: 10.1111/iju.70076
The Effectiveness and Safety Profile of Nivolumab-Plus-Ipilimumab in Previously Untreated Japanese Patients With Advanced or Metastatic Renal Cell Carcinoma (J-ENCORE Study)
Abstract
Objectives: Combination therapy of nivolumab-plus-ipilimumab has been approved for advanced or metastatic renal cell carcinoma in Japan, but large-scale clinical data targeting Japanese patients is limited. To evaluate the early outcomes and factors related to early progression and response.
Methods: J-ENCORE is an ongoing multicenter, prospective, observational study of the effectiveness and safety of nivolumab-plus-ipilimumab for patients in Japan with advanced or metastatic renal cell carcinoma. The objective response rate, duration of response, progression-free survival, overall survival, incidence of adverse events, and factors related to early progression within 3 months, and response were assessed.
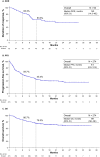
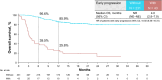
Results: We included 274 patients (median age: 68 years, 24.8% aged ≥ 75 years, 78.8% male). The median follow-up was 23.4 months. The objective response rate was 36.8%. Among responders, 63.3% had progression-free survival > 12 months. The median progression-free survival was 9.9 months; the 12-month overall survival was 76.3%. Of the patients, 77.0% experienced treatment-related adverse events, 42.3% experienced grade 3-4 events, and 1.1% experienced treatment-related death. Early progression was associated with female sex, poor risk status, liver metastasis, high baseline C-reactive protein levels, and high neutrophil-to-lymphocyte ratios. Responders were less likely to have bone metastases. Limitations include the observational nature of the study and a relatively short follow-up period.
Conclusions: This is the first prospective, real-world study to demonstrate the effectiveness and safety of nivolumab-plus-ipilimumab in Japan, with the results comparable to those of CheckMate 214. These findings support the use of nivolumab-plus-ipilimumab, although further studies with longer follow-up on nivolumab-plus-ipilimumab are needed.
Trail registration: ClinicalTrials.gov identifier: NCT04043975; University Hospital Medical Information Network-Clinical Trial Registration: UMIN000036772.
Keywords: Japan; ipilimumab; nivolumab; prospective study; renal cell carcinoma.
© 2025 The Author(s). International Journal of Urology published by John Wiley & Sons Australia, Ltd on behalf of The Japanese Urological Association.
Conflict of interest statement
Dr. Kimura reported receiving honoraria from Bristol Myers Squibb, Ono Pharmaceutical Co. Ltd., Merck Biopharma Co. Ltd., MSD K.K., Eisai Co. Ltd., Janssen Pharmaceutical K.K., Takeda Pharmaceutical Co. Ltd., Astellas Pharma Inc., Bayer Yakuhin Ltd., and Kissei Pharmaceutical Co. Ltd. Dr. Sazuka reported receiving honoraria from Bristol Myers Squibb, Ono Pharmaceutical Co. Ltd., and Takeda Pharmaceutical Co. Ltd. Dr. Hamamoto reported receiving honoraria from Ono Pharmaceutical Co. Ltd., MSD Co. Ltd., Pfizer Inc., and Eisai Co. Ltd. Dr. Nozawa reported receiving honoraria from Merck and Takeda Pharmaceutical Co. Ltd. Dr. Kondo reported receiving honoraria from Ono Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., and MSD K.K. Dr. Naito reported receiving honoraria from Bristol Myers Squibb, Ono Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Merck, Pfizer Japan Inc., MSD K.K., and Eisai Co. Ltd. Dr. Nagata reported receiving honoraria from Janssen Pharmaceutical K.K. and Sanofi K.K. Mr. Onodera is an employee of Ono Pharmaceutical Co. Ltd. Dr. Ito is an employee of Bristol Myers Squibb. Dr. Uemura reported receiving grants from Astra Zeneca K.K. and honoraria from Bristol Myers Squibb, Pfizer Japan Inc., Takeda Pharmaceutical Co. Ltd., Janssen Pharmaceutical K.K., and Sanofi K.K., and participated on an advisory board of Astra Zeneca K.K. and Janssen Pharmaceutical K.K. No other disclosures were reported. Dr. Naito is an Editorial Board member of the International Journal of Urology and a coauthor of this article. To minimize bias, they were excluded from all editorial decision‐making related to the acceptance of this article for publication.
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

