Effects of exercise on cognition and Alzheimer's biomarkers in a randomized controlled trial of adults with mild cognitive impairment: The EXERT study
- PMID: 40271888
- PMCID: PMC12019696
- DOI: 10.1002/alz.14586
Effects of exercise on cognition and Alzheimer's biomarkers in a randomized controlled trial of adults with mild cognitive impairment: The EXERT study
Abstract
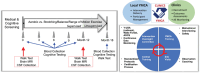
Introduction: The EXERT study (Exercise in Adults with Mild Memory Problems) was a Phase 3, multicenter, randomized controlled trial that examined effects of exercise on cognition and other measures of brain health in sedentary older adults with amnestic mild cognitive impairment (MCI).
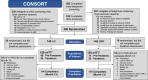
Methods: Participants were randomized to moderate-high intensity aerobic training (AX) or low-intensity stretching/balance/range of motion (SBR) for 18 months. Exercise was supervised for the first 12 months. Assessments were administered at baseline and every 6 months. The primary outcome was a global cognitive composite.
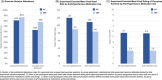
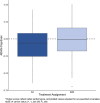
Results: A total of 296 participants were enrolled, and intervention adherence was high (supervised session attendance: AX = 81%, SBR = 87%). Intervention effects on cognition did not differ for AX and SBR (regression = -0.078, standard error [SE] = 0.074; p = 0.3). Notably, there was no 12 month cognition decline for either group, and mean 12 month hippocampal volume loss for both groups was low at 0.51%.
Discussion: Exercise intensity did not differentially affect cognitive trajectory. Intervention delivery was successful (high adherence) and cognition remained stable over 12 months for both MCI groups, an association that warrants further study.
Highlights: Exercise in Adults with Mild Memory Problems (EXERT) was a large multisite randomized controlled trial of moderate-high intensity aerobic training versus lower-intensity flexibility and balance exercise in sedentary older adults with amnestic mild cognitive impairment (MCI). A sensitive and validated measure of global cognitive function, the Alzheimer's Disease Assessment Scale-Cognition supplemented with tests of executive function (ADAS-Cog-Exec), was used to assess intervention efficacy with 12 months of supervised exercise. There was no intervention group difference on the 12-month cognitive trajectory of the ADAS-Cog-Exec. Intervention delivery was successful (high adherence), and cognition remained stable over 12 months for both exercise groups. Regular supported moderate-high or lower-intensity exercise may stall decline in adults with amnestic MCI, but further investigation is needed.
Keywords: Alzheimer's; brain MRI; clinical trial; cognition; exercise; mild cognitive impairment; multisite; nonpharmacological intervention.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Feldman receives grant funding from the National Institute on Aging (U19AG010483‐22), from Biohaven Pharmaceuticals, Vivoryon (Probiodrug), and LuMind Foundation; service agreements for consulting activities with LuMind, Axon Neuroscience, Novo Nordisk, Arrowhead Pharmaceuticals, Roche/Genentech Pharmaceuticals (DMC/DSMB), Tau Consortium (SAB), and Janssen Research & Development (DSMB); support for travel from Novo Nordisk, Royal Society of Canada, Translating Research in Elder Care (TREC), Association for Frontotemporal Dementia (AFTD), and Rainwater Charitable Foundation; and a philanthropic donation for the Epstein Family Alzheimer Research Collaboration. No personal funds have been received for these activities. Feldman personally receives royalties for patent: Feldman HH (filed November 26, 2008). Detecting and Treating Dementia Serial Number 12/3‐2691 U.S. Patent No. PCT/US2007/07008. Washington, DC: U.S. Patent and Trademark Office. For other authors, there are no conflicts to report for this work. Author disclosures are available in the Supporting Information.
Figures
References
-
- Prince M, Wimo A, Guerchet M, Ali GC, Wu Y. The Global Impact of Dementia: an Analysis of Prevalence, Incidence, Cost and Trends. Alzheimer's Disease International; 2015.
-
- Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379‐2388. - PubMed
-
- Winblad B, Gauthier S, Scinto L, et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology. 2008;70(22):2024‐2035. - PubMed
-
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388(1):9‐21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

