Epidemiology and evolutionary dynamics of H9N2 avian influenza virus in Bangladesh
- PMID: 40271995
- PMCID: PMC12123974
- DOI: 10.1080/22221751.2025.2498574
Epidemiology and evolutionary dynamics of H9N2 avian influenza virus in Bangladesh
Abstract
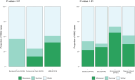
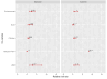
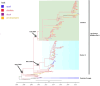
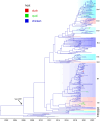
Low pathogenicity avian influenza (LPAI) H9N2 has been enzootic in Bangladeshi poultry since 2006. H9N2 outbreaks can decrease egg production and growth and pose a risk to human health. The role of avian hosts in the persistence, evolution, and dispersion of H9N2 is poorly understood in Bangladesh. Hence, this study unveils the intricate role of major host species in virus maintenance and evolution and the temporal and seasonal patterns of H9N2 in Bangladesh from 2006 to 2023. Multinomial logistic regression analysis indicated that the circulation of H9N2 in different species and interfaces is significantly influenced by the seasons. Bayesian phylogenetic analysis of H9N2 sequences in Bangladesh revealed two distinct lineages: G1 and Eurasian. The G1 lineage split into two clusters, coexisting until 2019, at which point only one cluster persisted. Bayesian phylodynamic analysis of G1 lineage unveiled frequent bidirectional viral transitions among ducks, chickens, and quails. Chickens might be a pivotal source of H9N2 in Bangladesh, with a higher number of viral transitions from chickens to ducks and quails. Quails appear to acquire most of their viral transitions from chickens rather than ducks, suggesting that quail epizootics are primarily triggered by spillover events from chickens. Our results suggest viral circulation in commercial chickens despite vaccination. The vaccination approach should be revised, assess vaccine efficacy, and extension of vaccination to backyard chickens and quails.
Keywords: AIV; LPAI; Phylodynamics; poultry; surveillance; vaccination; zoonotic.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
