Burkholderia cenocepacia and Pseudomonas aeruginosa in dual-species models: Insights into population distribution, antibiotic susceptibility, and virulence
- PMID: 40272017
- PMCID: PMC12036490
- DOI: 10.1080/21505594.2025.2494039
Burkholderia cenocepacia and Pseudomonas aeruginosa in dual-species models: Insights into population distribution, antibiotic susceptibility, and virulence
Abstract
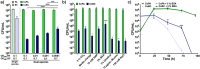
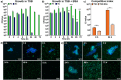
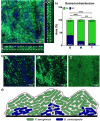
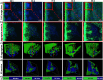
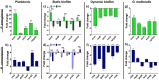
Multispecies biofilms are communities composed of different microorganisms embedded in an auto-synthesized polymeric matrix. Pseudomonas aeruginosa and Burkholderia cenocepacia are two multidrug-resistant and biofilm-forming opportunistic pathogens often found in the lungs of people living with cystic fibrosis. In this context, planktonic, static, and dynamic biofilms and in vivo models of both species were optimized in this work to understand their population dynamics, disposition, virulence, and antibiotic susceptibility. From the coculture models optimized in this work, we determined that B. cenocepacia grows in a clustered, aggregative manner at the bottom layers of biofilms, in close contact with P. aeruginosa, that tends to occupy the top layers. Their coexistence increases virulence-related gene expression in both species at early stages of coinfection and in in vivo models, while there was a general downregulation of virulence-related genes after longer coexistence periods as they eventually reach a non-competitive stage during chronic infections. When evaluating antimicrobial susceptibility, a decrease of antimicrobial tolerance was observed in both species when co-cultured. These findings shed light on the differential behavior of P. aeruginosa and B. cenocepacia in dual-species systems, stressing the relevance of multispecies studies in the clinical context.
Keywords: Polymicrobial biofilm; antibiotic; biofilm spatial distribution; coinfection; gene expression; virulence.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
