Newly diagnosed heart failure with reduced ejection fraction: timing, sequencing, and titration of guideline-recommended medical therapy
- PMID: 40272103
- PMCID: PMC12208773
- DOI: 10.1093/eurheartj/ehaf244
Newly diagnosed heart failure with reduced ejection fraction: timing, sequencing, and titration of guideline-recommended medical therapy
Abstract
Background and aims: Despite guidelines recommending rapid initiation and up-titration of Guideline-recommended medical therapy (GRMT) for heart failure (HF) with reduced ejection fraction (HFrEF), its feasibility in daily practice remains unclear. TITRATE-HF studies the feasibility of rapid GRMT implementation in de novo HFrEF patients, investigating titration patterns and identifying barriers to effective treatment.
Methods: This analysis focuses on the de novo HFrEF patients included in the TITRATE-HF study, an ongoing prospective HF registry conducted in 48 Dutch hospitals. A detailed logbook for each GRMT drug class was recorded, from diagnosis to six months, including initiations, dose adjustments, discontinuations, and reasons for changes.
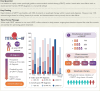
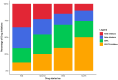
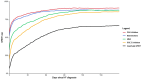
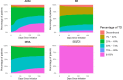
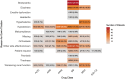
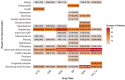
Results: The study included 1508 de novo HFrEF patients (median age: 70 years [inter-quartile ranges, IQR 62-77]; 31% women; median left ventricular ejection fraction: 30% [IQR 25-35]). At 6 weeks, 46% of patients were using quadruple therapy. Within 6 weeks post-HFrEF diagnosis, 50% of patients were prescribed quadruple therapy at some point, with 84% remaining on it after 180 days. At 6 months, 66.3% of patients were prescribed quadruple therapy, but only 1.3% achieved target doses for all four drug classes. While side effects accounted for 20%-37% of cases where target doses were not reached, a large proportion was attributed to physicians accepting suboptimal doses. Drug titrations occurred frequently in the first 60 days after diagnosis, fading afterwards. Discontinuation rates for angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, angiotensin receptor-neprilysin inhibitors, beta-blocker, mineralocorticoid receptor antagonists, and sodium-glucose cotransporter 2 inhibitors were 13%, 11%, 9%, 9%, 11%, and 9%, respectively, primarily due to side effects or intolerances. Rechallenging these drug classes was successful in over 83% of patients.
Conclusions: The TITRATE-HF study demonstrates that rapid initiation of GRMT for HFrEF is feasible in real-world clinical practice. Nonetheless, our results highlight the urgency for a proactive approach and ongoing dose titration of pharmacological therapy beyond the initial first months to fully optimize treatment.
Keywords: Guidelines; Implementation; Pharmacotherapy; Registry; Sequencing; Titration.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures
References
-
- Mebazaa A, Davison B, Chioncel O, Cohen-Solal A, Diaz R, Filippatos G, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet 2022;400:1938–52. 10.1016/S0140-6736(22)02076-1 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

