Relationship Between Best Tumor Shrinkage and Progression-Free Survival and Overall Survival in Patients With Progressive Midgut Neuroendocrine Tumors Treated With [177Lu]Lu-DOTA-TATE: Ad Hoc Analysis of the Phase III NETTER-1 Trial
- PMID: 40272146
- PMCID: PMC12020026
- DOI: 10.1002/cam4.70744
Relationship Between Best Tumor Shrinkage and Progression-Free Survival and Overall Survival in Patients With Progressive Midgut Neuroendocrine Tumors Treated With [177Lu]Lu-DOTA-TATE: Ad Hoc Analysis of the Phase III NETTER-1 Trial
Abstract
Background: In many solid tumors, early tumor shrinkage predicts the durability of treatment response. It is unclear whether this is the case for neuroendocrine tumors treated with peptide receptor radionuclide therapy (PRRT).
Methods: Data from the phase III NETTER-1 study of [177Lu]Lu-DOTA-TATE (177Lu-DOTATATE) for the treatment of advanced, well-differentiated, midgut NETs were used to investigate whether objective tumor shrinkage (local review) with 177Lu-DOTATATE is associated with progression-free survival (PFS) and overall survival (OS) duration.
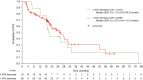
Results: Overall, 117 patients were treated with 177Lu-DOTATATE (four cycles of 7.4 GBq every 8 weeks). In a landmark analysis, best tumor shrinkage from baseline until data cut-off (prior to first progression) was not associated with PFS (n = 102; hazard ratio: 1.002 [95% confidence interval (CI): 0.99-1.02]; nominal p = 0.7808). In further ad hoc analyses, patients on the 177Lu-DOTATATE arm were dichotomized into ≥ 30% tumor shrinkage from baseline (18/117 [15.4%]) and < 30% shrinkage (99/117 [84.6%]). Median (95% CI) PFS was 17.6 (16.5-30.3) months in the ≥ 30% shrinkage group and 25.0 (19.4-31.0) months in the < 30% group. OS was not significantly different for the two tumor shrinkage groups (not estimable [31.0 months-not estimable] and 44.3 [34.9-53.8] months, respectively).
Conclusions: These results suggest the benefit of PRRT and the potential PFS and OS benefit of 177Lu-DOTATATE should not be based on tumor shrinkage (objective response versus stable disease) and that lack of tumor shrinkage should not impact application of the approved four cycles of 177Lu-DOTATATE.
Keywords: [177Lu]Lu‐DOTA‐TATE; neuroendocrine tumor; overall survival; progression‐free survival; tumor shrinkage.
© 2025 Advanced Accelerator Applications and The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Marianne Pavel: Received honoraria for presentations and/or acted as a consultant for Advanced Accelerator Applications, a Novartis Company; Ipsen; Novartis Pharma AG; Eli Lilly; Riemser; Boehringer Ingelheim; MSD; Hutchmed; Serb; Sanofi; Esteve; Tairix; and ITM Radiopharma, and serves as an adviser for SMC of Crinetics and SSC of Novartis. Martyn E. Caplin: Received honoraria for presentations and/or acted as a consultant for Advanced Accelerator Applications, a Novartis Company; Crinetics; Ipsen; and Pfizer. Philippe Ruszniewski: Scientific adviser to Advanced Accelerator Applications, a Novartis Company; Ipsen; and ITM Radiopharma. Marianna Hertelendi: Full‐time employee of Novartis Basel and holds shares in Novartis. Eric P. Krenning: Retired (Erasmus MC, Rotterdam, Netherlands)—previous shareholder of Advanced Accelerator Applications, a Novartis Company/BioSynthema. Jonathan R. Strosberg: Consulted for Novartis; institutional trial support from ITM and Radiomedix.
Figures



References
-
- Eisenhauer E. A., Therasse P., Bogaerts J., et al., “New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1),” European Journal of Cancer 45 (2009): 228–247. - PubMed
-
- Therasse P., Arbuck S. G., Eisenhauer E. A., et al., “New Guidelines to Evaluate the Response to Treatment in Solid Tumors,” Journal of the National Cancer Institute 92 (2000): 205–216. - PubMed
-
- Ballesio L., Gigli S., Di Pastena F., et al., “Magnetic Resonance Imaging Tumor Regression Shrinkage Patterns After Neoadjuvant Chemotherapy in Patients With Locally Advanced Breast Cancer: Correlation With Tumor Biological Subtypes and Pathological Response After Therapy,” Tumour Biology 39 (2017): 1010428317694540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

