SARS-CoV-2 biological clones are genetically heterogeneous and include clade-discordant residues
- PMID: 40272156
- PMCID: PMC12090815
- DOI: 10.1128/jvi.02250-24
SARS-CoV-2 biological clones are genetically heterogeneous and include clade-discordant residues
Abstract
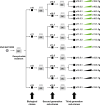
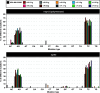
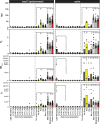
Defective genomes are part of SARS-CoV-2 quasispecies. High-resolution, ultra-deep sequencing of bulk RNA from viral populations does not distinguish RNA mutations, insertions, and deletions in viable genomes from those in defective genomes. To quantify SARS-CoV-2 infectious variant progeny, virus from four individual plaques (biological clones) of a preparation of isolate USA-WA1/2020, formed on Vero E6 cell monolayers, was subjected to further biological cloning to yield 9 second-generation and 15 third-generation sub-clones. Consensus genomic sequences of the biological clones and sub-clones included an average of 2.8 variations per viable genome, relative to the consensus sequence of the parental USA-WA1/2020 virus. This value is 6.5-fold lower than the estimates for biological clones of other RNA viruses such as bacteriophage Qβ, foot-and-mouth disease virus, or hepatitis C virus in cell culture. The mutant spectrum complexity of the nsp12 (polymerase)- and spike (S)-coding region was unique in the progeny of each of 10 third-generation sub-clones; they shared 2.4% of the total of 164 different mutations and deletions scored in the 3,719 genomic residues that were screened. The presence of minority out-of-frame deletions revealed the ease of defective genome production from an individual infectious genome. Several low-frequency point mutations and deletions were clade-discordant in that they were not typical of USA-WA1/2020 but served to define the consensus sequences of future SARS-CoV-2 clades. Implications for SARS-CoV-2 adaptability and COVID-19 control of the viable genome heterogeneity and the generation of complex mutant spectra from individual genomes are discussed.IMPORTANCESequencing of biological clones is a means to identify mutations, insertions, and deletions located in viable genomes. This distinction is particularly important for viral populations, such as those of SARS-CoV-2, that contain large proportions of defective genomes. By sequencing biological clones and sub-clones, we quantified the heterogeneity of the viable complement of USA-WA1/2020 to be lower than exhibited by other RNA viruses. This difference may be due to a reduced mutation rate or to limited tolerance of the large coronavirus genome to incorporate mutations and deletions and remain functional or a combination of both influences. The presence of clade-discordant residues in the progeny of individual biological sub-clones suggests limitations in the occupation of sequence space by SARS-CoV-2. However, the complex and unique mutant spectra that are rapidly generated from individual genomes suggest an aptness to confront selective constraints.
Keywords: RNA virus; defective viral genome; deletion; diversity indices; mutant spectrum; point mutation; population complexity; quasispecies; ultra-deep sequencing; viral clade.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

