Unraveling the interplay between mesenchymal stem cells, gut microbiota, and systemic sclerosis: therapeutic implications
- PMID: 40272189
- PMCID: PMC12131792
- DOI: 10.1128/spectrum.01576-24
Unraveling the interplay between mesenchymal stem cells, gut microbiota, and systemic sclerosis: therapeutic implications
Abstract
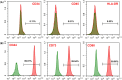
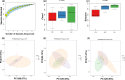
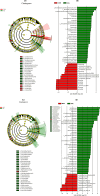
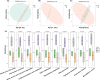
Systemic sclerosis (SSc) is an autoimmune disease with progressive fibrotic disorders in multiple organs. Mesenchymal stem cells (MSCs) have shown great potential in treating SSc, but the exact regulatory mechanism is not fully understood. In this study, we used human umbilical cord-derived MSCs (hUC-MSCs) to treat SSc mice induced by bleomycin. The gut microbiota composition and predicted functions were analyzed using 2bRAD sequencing of fecal samples from control, SSc, and MSCs-treated mice. Treatment with MSCs improved the bleomycin-induced SSc mice, characterized by significantly reduced collagen deposition and dermal thickness. The gut microbiota of SSc mice exhibited lower species evenness and was clearly separated from the control mice based on beta diversity. MSC treatment led to a significant reduction of conditionally pathogenic bacteria enriched in SSc, including Akkermansia muciniphila and Parasutterella excrementihominis. Conversely, the relative abundance of butyrate-producing bacteria, such as Roseburia, Butyricicoccus porcorum, and Gemmiger formicilis, was notably increased in MSCs-treated SSc mice. Additionally, the functional analysis revealed that MSCs intervention effectively enhanced sulfur metabolism, tryptophan metabolism, citrate cycle, RNA polymerase, and beta-lactam resistance. In summary, the findings in the present study have suggested the close association between gut microbiota and metabolic dysbiosis in mice with SSc. The administration of MSCs has been shown to regulate the disrupted metabolic pathways in SSc mice, thus restoring the normal function of the gut microbiota. This study provides valuable insights into the specific gut microbiota and metabolic pathways involved in the efficacy of MSC treatment, thereby proposing a novel therapeutic strategy for SSc.
Importance: Human umbilical cord-derived mesenchymal stem cells (HUC‑MSCs) demonstrate efficacy in alleviating skin thickening and collagen deposition in systemic sclerosis (SSc) mice, which also regulate the gut microbiota composition and function. Specifically, MSC intervention leads to a notable increase in butyrate-producing bacteria, a decrease in Akkermansia muciniphila and Parasutterella excrementihominis, and a reversal of the dysregulated microbial function in SSc mice. These findings underscore the potential significance of gut microbiota in the therapeutic effects of MSCs in SSc.
Keywords: 2bRAD sequence; gut microbiota; mesenchymal stem cells; metabolism; systemic sclerosis.
Conflict of interest statement
The authors declare no conflict of interest
Figures


Similar articles
-
The role of the microbiota and metabolites in the treatment of pulmonary fibrosis with UC-MSCs: Integrating fecal metabolomics and 16S rDNA analysis.PLoS One. 2025 Jan 9;20(1):e0313989. doi: 10.1371/journal.pone.0313989. eCollection 2025. PLoS One. 2025. PMID: 39787138 Free PMC article.
-
Exploring the role of gut microbiota modulation in the long-term therapeutic benefits of early MSC transplantation in MRL/lpr mice.Cell Mol Biol Lett. 2025 Apr 18;30(1):49. doi: 10.1186/s11658-025-00716-8. Cell Mol Biol Lett. 2025. PMID: 40251524 Free PMC article.
-
Antifibrotic effects and mechanisms of mesenchymal stem cell-derived exosomes in a systemic sclerosis mouse model: Possible contribution of miR-196b-5p.J Dermatol Sci. 2021 Oct;104(1):39-47. doi: 10.1016/j.jdermsci.2021.08.006. Epub 2021 Aug 26. J Dermatol Sci. 2021. PMID: 34479773
-
Impact of Mesenchymal Stem Cells on the Gut Microbiota and Microbiota Associated Functions in Inflammatory Bowel Disease: A Systematic Review of Preclinical Evidence on Animal Models.Curr Stem Cell Res Ther. 2024;19(7):981-992. doi: 10.2174/011574888X250413230920051715. Curr Stem Cell Res Ther. 2024. PMID: 37817517
-
Mesenchymal stem cell-based therapy for autoimmune-related fibrotic skin diseases-systemic sclerosis and sclerodermatous graft-versus-host disease.Stem Cell Res Ther. 2023 Dec 18;14(1):372. doi: 10.1186/s13287-023-03543-w. Stem Cell Res Ther. 2023. PMID: 38111001 Free PMC article. Review.
Cited by
-
Stem cell therapy in systemic sclerosis.Clin Rheumatol. 2025 Aug;44(8):3139-3151. doi: 10.1007/s10067-025-07557-y. Epub 2025 Jul 2. Clin Rheumatol. 2025. PMID: 40601079 Free PMC article. Review.
-
Harmonizing the Gut Microbiome and Cellular Immunotherapies: The Next Leap in Cancer Treatment.Cells. 2025 May 14;14(10):708. doi: 10.3390/cells14100708. Cells. 2025. PMID: 40422211 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

