Type 1 Diabetes TrialNet: Leading the Charge in Disease Prediction, Prevention, and Immunotherapeutic Mechanistic Understanding
- PMID: 40272284
- PMCID: PMC12178618
- DOI: 10.2337/dc24-2908
Type 1 Diabetes TrialNet: Leading the Charge in Disease Prediction, Prevention, and Immunotherapeutic Mechanistic Understanding
Abstract
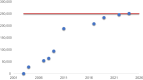
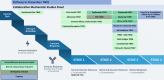
Established by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) in 2001, Type 1 Diabetes TrialNet (TrialNet) is an international consortium of clinical research centers that studies the development of type 1 diabetes and performs clinical studies aimed at delaying or preventing the disease. In recognition of NIDDK's 75th anniversary, this review will summarize the major findings, accomplishments, and future opportunities of its long-running program TrialNet. More than 20 intervention, observational, and mechanism-directed clinical studies have been conducted in collaboration with thousands of people living with type 1 diabetes and their families. New and repurposed immunotherapies have been successful in stages 2 and 3 of type 1 diabetes, contributing to the U.S. Food and Drug Administration approval of the first disease-modifying therapy to delay the onset of type 1 diabetes. Mechanistic findings continue to drive ongoing and future trial designs including novel combination therapies. TrialNet has several ongoing trials, with several for early stages of type 1 diabetes in development. There are new initiatives within TrialNet for community engagement, increasing clinical trial representation, personalizing treatments, and training the next generation of translational investigators.
© 2025 by the American Diabetes Association.
Conflict of interest statement
Figures

References
-
- Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 1965;14:619–633 - PubMed
Publication types
MeSH terms
Grants and funding
- U01 DK085476/DK/NIDDK NIH HHS/United States
- U01 DK085453/DK/NIDDK NIH HHS/United States
- NH/NIH HHS/United States
- U01 DK103282/DK/NIDDK NIH HHS/United States
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- UC4 DK106993/DK/NIDDK NIH HHS/United States
- National Institute of Allergy and Infectious Diseases (NIAID)
- U01 DK085509/DK/NIDDK NIH HHS/United States
- National Institutes of Health (NIH)
- U01 DK107014/DK/NIDDK NIH HHS/United States
- U01 DK085466/DK/NIDDK NIH HHS/United States
- U01 DK061058/DK/NIDDK NIH HHS/United States
- U01 DK106993/DK/NIDDK NIH HHS/United States
- U01 DK107013/DK/NIDDK NIH HHS/United States
- UC4 DK106993/DK/NIDDK NIH HHS/United States
- U01 DK085509/DK/NIDDK NIH HHS/United States
- U01 DK106984/DK/NIDDK NIH HHS/United States
- U01 DK106994/DK/NIDDK NIH HHS/United States
- U01 DK061042/DK/NIDDK NIH HHS/United States
- U01 DK107013/DK/NIDDK NIH HHS/United States
- U01 DK106984/DK/NIDDK NIH HHS/United States
- U01 DK107014/DK/NIDDK NIH HHS/United States
- U01 DK106994/DK/NIDDK NIH HHS/United States
- U01 DK084565/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- U01 DK103282/DK/NIDDK NIH HHS/United States
- NH/NIH HHS/United States
- U01 DK061042/DK/NIDDK NIH HHS/United States
- U01 DK085504/DK/NIDDK NIH HHS/United States
- U01 DK085476/DK/NIDDK NIH HHS/United States
- U01 DK085461/DK/NIDDK NIH HHS/United States
- U01 DK061058/DK/NIDDK NIH HHS/United States
- UC4 DK085466/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- U01 DK085461/DK/NIDDK NIH HHS/United States
- U01 DK085453/DK/NIDDK NIH HHS/United States
- U01 DK085504/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

