Safety and efficacy of continuous infusion terlipressin (BIV201): A phase 2 trial in patients with decompensated cirrhosis and refractory ascites
- PMID: 40272365
- PMCID: PMC12445179
- DOI: 10.1097/LVT.0000000000000623
Safety and efficacy of continuous infusion terlipressin (BIV201): A phase 2 trial in patients with decompensated cirrhosis and refractory ascites
Abstract
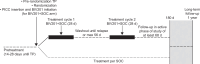
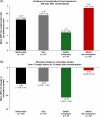
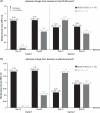
Refractory ascites often requires therapeutic paracentesis, which is associated with potential risks and diminished quality of life. Terlipressin is a vasopressin analog that is indicated for i.v. bolus injection for hepatorenal syndrome, with the potential to reduce large-volume ascites and its complications. Continuous infusion of terlipressin is associated with fewer adverse effects than bolus dosing. The efficacy and safety of continuous infusion of a novel liquid formulation of terlipressin acetate (BIV201) were evaluated in this open-label phase 2 study. Patients with cirrhosis and refractory ascites were randomly assigned (2:1) to receive two 28-day cycles of continuous infusion BIV201 plus standard of care (SOC) separated by a ≤56-day washout (n=10), or SOC alone (n=5). Data analysis was limited by the small sample size and confounded by a potential interaction with gabapentinoids in the BIV201+SOC group. Nonetheless, there were differences in favor of BIV201+SOC versus SOC in the coprimary efficacy endpoints and several quality of life assessments. The beneficial effects of BIV201 on liver complications (mean: 90% CI; BIV201-completers=2.87: 1.51; 5.46 vs. SOC=2.38: 1.20; 4.73) and the change in cumulative ascites (mean: 90% CI; BIV201-completers=-10.76: -26.51; 5.00 vs. SOC=-4.99: -21.95; 11.97) were more pronounced versus SOC in the 5 BIV201+SOC patients who completed both treatment cycles. There were also greater improvements in exploratory quality of life assessments and the percent change in therapeutic paracenteses with BIV201+SOC (-27.94±41.80) versus SOC (-16.67±45.64). Despite the high rate of hyponatremia in the BIV201+SOC group (4/10 patients), the safety profile suggested that continuous BIV201 infusion was well tolerated. These findings support further development of BIV201 in confirmatory trials.
Keywords: ascites; decompensated cirrhosis; hyponatremia; outpatient; phase 2 clinical trial; terlipressin.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Jasmohan S. Bajaj received grants from Bausch, Sequana, Cosmo, BioVie Inc., Mallinckrodt, and Grifols. Ethan M. Weinberg consults for Mallinckrodt, BioVie Inc., and PharmaIN. K. Rajender Reddy advises and received grants from Mallinckrodt, BioVie Inc., and Sequana. He received grants from GSK Grifols, Exact Sciences, BMS, BD Therapeutics, Camarus, Intercept, Regeneron, HCC-TARGET, and NASH-TARGET paid to the University of Pennsylvania. He advises Pfizer, Novo Nordisk, and Spark Therapeutics. He has other interests with Novartis, Genkyotex, AstraZeneca, HiTide, Gastroenterology, and Hepatology Reports. He received royalties from UpToDate. Andrew P. Keaveny advises BioVie Inc. and HepQuant LLC. Michael K. Porayko consults for BioVie Inc., River2Renal, and PharmaIN. David Koch consults for and received grants from BioVie. He advises and is on the speakers’ bureau for Madrigal. He received grants from Zydus and Target RWE. Paul J. Thuluvath is on the speakers’ bureau and has received grants from AbbVie and Gilead. He is on the speakers’ bureau for Mallinckrodt and UpToDate. He received grants from NIH Recover, Bayer, Bristol Myers Squibb, Cirius Therapeutics, Cymabay, Intercept, Novo Nordisk, Target, Exact Sciences, Roche, BioVie Inc., NGM Bio, Viking, Hanmi, Salix, Alnylam, Inventiva, Corrona, AstraZeneca, HighTide Biopharma, Akero, Madrigal, Ipsen, Syntis Bio, and 89bio. Douglas A. Simonetto consults for Mallinckrodt, BioVie Inc., Evive, Resolution Therapeutics, AstraZeneca, Iota, and PharmaIN. Paolo Angeli consults for and owns intellectual property rights in BioVie Inc. He is on the speakers’ bureau and received grants from Grifols. He is on the speakers’ bureau for CLS Behring. Eric S. Orman advises Biovie. He consults for Salix. Jeffrey Zhang consults for BioVie Inc. Susan Clausen consults for BioVie Inc. Elisa Dauphinée consults for BioVie Inc. Joseph M. Palumbo received a grant from the U.S. Department of Defense (DOD), awarded through the Peer Reviewed Medical Research Program (PRMRP) of the Congressionally Directed Medical Research Programs (CDMRP). He is employed by, consults for, and advises Psychae Therapeutics Pty Ltd, Phocus, and Stalicla, SA. He is employed by Aozora Science LLC. He is employed by and owns stock in BioVie Inc. He owns intellectual property rights in Zynerba and Mitsubishi Tanabe Pharma Corporation. He has other interests in Merck Research Laboratories. Penelope Markham is employed by and owns stock in BioVie Inc.
Figures


References
-
- Biggins SW, Angeli P, Garcia-Tsao G, Ginès P, Ling SC, Nadim MK, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014–1048. - PubMed
-
- Will V, Rodrigues SG, Berzigotti A. Current treatment options of refractory ascites in liver cirrhosis—A systematic review and meta-analysis. Dig Liver Dis. 2022;54:1007–1014. - PubMed
-
- Arroyo V, Ginés P, Planas R, Panés J, Rodés J. Management of patients with cirrhosis and ascites. Semin Liver Dis. 1986;6:353–369. - PubMed
-
- Cárdenas A, Arroyo V. Refractory ascites. Dig Dis. 2005;23:30–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

