Botulinum toxin (A)-induced bone loss is associated with increased blood velocity and reduced vascular bone porosity
- PMID: 40272396
- PMCID: PMC12685722
- DOI: 10.1093/jbmr/zjaf057
Botulinum toxin (A)-induced bone loss is associated with increased blood velocity and reduced vascular bone porosity
Abstract
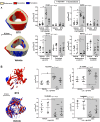
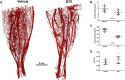
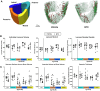
Disuse-induced bone loss is a common consequence of spaceflight and prolonged bed rest. Intraosseous blood vessel volume and number are decreased in rodents after sciatic nerve resection, and femoral and tibial perfusion and blood flow to the femoral shaft and marrow are reduced after hindlimb unloading. However, it is unclear if alterations in the flow of blood contribute to botulinum toxin (BTX)-induced bone loss. The objective of this study was to assess patterns of tibial bone loss and alterations in blood flow in murine hindlimbs following BTX injection. We hypothesize that flow of blood to the affected hindlimb will diminish along with bone mass and structure. Skeletally mature C57Bl/6J female were injected with BTX (n = 15) or vehicle (n = 14). Paralysis was confirmed using digit abduction, wire hang tests, and activity analysis. In vivo microCT and ex vivo synchrotron tomography were used to assess bone mass, microstructure, (re)modeling, as well as vascular and lacunar porosity. Blood flow in the hindlimbs and cardiac structure/function was monitored by echocardiography. After 3 wk, BTX-injected tibiae had 16% lower cortical thickness and 66% lower trabecular bone volume fraction compared to baseline. MicroCT-based timelapse morphometry showed bone loss was predominantly at endocortical surfaces. Bone loss in the contralateral limb was coincident with reduced rearing capability of BTX-injected mice compared to vehicle controls. Bony vascular canal thickness and surface area were reduced, but there was no change in lacunar properties due to BTX. In vivo ultrasound demonstrated increased velocity time integral for blood flow due to BTX injection in femoral and popliteal but not in saphenous arteries. Thus, BTX led to significant bone loss in hindlimbs, while increasing blood velocity in the femoral popliteal arteries and decreasing vascular porosity. The vascular response to BTX differs from what has been observed in other hindlimb unloading models.
Keywords: blood flow; bone loss; botulinum toxin A; mechanical unloading; osteocyte network analysis; synchrotron imaging; vascular porosity.
Plain language summary
Bone deteriorates when not used, such as in spaceflight, long-term bed rest or paralysis. To examine changes in blood supply to bone during disuse, we paralyzed the muscles in mice using botulinum toxin (commonly known as Botox). For 3 weeks, we monitored blood flow in the paralyzed leg and imaged bone structure. The blood flow was high in the arteries of Botox injected limbs, but vascular channels were reduced inside the bone. Bone loss was evident and mostly occurred on the inner bone surface. Understanding paralysis-induced vasculature changes may help to prevent and treat bone loss in these conditions.
© The Author(s) 2025. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research.
Conflict of interest statement
None of the authors have any perceived or actual conflicts of interest to report.
Figures





Comment in
-
Bone loss, botulinum toxin, and cardiovascular function.J Bone Miner Res. 2025 Nov 29;40(12):1315-1316. doi: 10.1093/jbmr/zjaf137. J Bone Miner Res. 2025. PMID: 41051364 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

