Enhancing Cold Adaptation of Bidomain Amylases by High-Throughput Computational Engineering
- PMID: 40272877
- PMCID: PMC12258674
- DOI: 10.1002/anie.202505991
Enhancing Cold Adaptation of Bidomain Amylases by High-Throughput Computational Engineering
Abstract
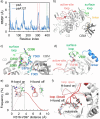
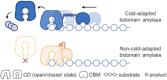
Cold-adapted bidomain enzymes have the potential to foster industrial sustainability by reducing energy consumption and greenhouse gas emissions. Despite their allure, these benefits are unattainable, as the molecular basis of cold adaptation remains elusive, and there are no strategies to guide the acquisition of this behavior. To uncover principles of cold adaptation, we selected the cold-adapted Saccharophagus degradans amylase (sdA) and mesophilic Pseudomonas saccharophila amylase (psA) as model systems. Through molecular dynamics (MD) simulations and biochemical assays, we found that sdA exhibits significantly greater interdomain separation between its catalytic domain (CD) and carbohydrate-binding module (CBM) at low temperatures. Therefore, we introduce the domain separation index metric to guide the in silico screening of 120 psA variants using high-throughput enzyme modeling. The highest-ranked variant, psA121, shows a 3-fold increase in relative activity over the wild type at 0 °C. MD simulations suggest that psA121 achieves cold adaptation via helical linkers, which induce interdomain separation and enhance flexibility of the active site and binding loops via dynamic allostery, promoting substrate recruitment, binding, and catalysis at lower temperatures. This study highlights how domain separation contributes to cold adaptation in bidomain amylases and offers strategies for introducing such cold adaptation to other systems.
Keywords: Bidomain amylase; Cold adaptation; EnzyHTP; Enzyme engineering; High‐throughput virtual screening; Linker engineering.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

