Ketogenesis mitigates metabolic dysfunction-associated steatotic liver disease through mechanisms that extend beyond fat oxidation
- PMID: 40272888
- PMCID: PMC12165805
- DOI: 10.1172/JCI191021
Ketogenesis mitigates metabolic dysfunction-associated steatotic liver disease through mechanisms that extend beyond fat oxidation
Abstract
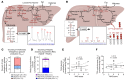
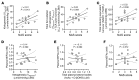
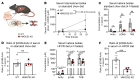
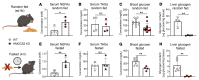
The progression of metabolic dysfunction-associated steatotic liver disease (MASLD) to metabolic dysfunction-associated steatohepatitis (MASH) involves alterations in both liver-autonomous and systemic metabolism that influence the liver's balance of fat accretion and disposal. Here, we quantify the contributions of hepatic oxidative pathways to liver injury in MASLD-MASH. Using NMR spectroscopy, UHPLC-MS, and GC-MS, we performed stable isotope tracing and formal flux modeling to quantify hepatic oxidative fluxes in humans across the spectrum of MASLD-MASH, and in mouse models of impaired ketogenesis. In humans with MASH, liver injury correlated positively with ketogenesis and total fat oxidation, but not with turnover of the tricarboxylic acid cycle. Loss-of-function mouse models demonstrated that disruption of mitochondrial HMG-CoA synthase (HMGCS2), the rate-limiting step of ketogenesis, impairs overall hepatic fat oxidation and induces an MASLD-MASH-like phenotype. Disruption of mitochondrial β-hydroxybutyrate dehydrogenase (BDH1), the terminal step of ketogenesis, also impaired fat oxidation, but surprisingly did not exacerbate steatotic liver injury. Taken together, these findings suggest that quantifiable variations in overall hepatic fat oxidation may not be a primary determinant of MASLD-to-MASH progression, but rather that maintenance of ketogenesis could serve a protective role through additional mechanisms that extend beyond overall rates of fat oxidation.
Keywords: Fatty acid oxidation; Hepatology; Intermediary metabolism; Metabolism; Obesity.
Conflict of interest statement
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

