The gut microbiome enhances breast cancer immunotherapy following bariatric surgery
- PMID: 40272913
- PMCID: PMC12220976
- DOI: 10.1172/jci.insight.187683
The gut microbiome enhances breast cancer immunotherapy following bariatric surgery
Abstract
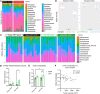
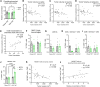
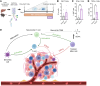
Bariatric surgery is associated with improved breast cancer (BC) outcomes, including greater immunotherapy effectiveness in a preclinical BC model. A potential mechanism of bariatric surgery-associated protection is the gut microbiota. Here, we demonstrate the dependency of improved immunotherapy response on the post-bariatric surgery gut microbiome via fecal microbiota transplantation (FMT). Response to αPD-1 immunotherapy was significantly improved following FMT from formerly obese bariatric surgery-treated mice. When stool from post-bariatric surgery patients was transplanted into recipient mice and compared to the patients' presurgery transplants, postsurgery microbes significantly reduced tumor burden and doubled immunotherapy effectiveness. Microbes impact tumor burden through microbially derived metabolites, including branched-chain amino acids (BCAAs). Circulating BCAAs correlated significantly with natural killer T (NKT) cell content in the tumor microenvironment in donor mice after bariatric surgery and FMT recipients of donor cecal content after bariatric surgery compared with obese controls. BCAA supplementation replicated improved αPD-1 effectiveness in 2 BC models, supporting the role of BCAAs in increased immunotherapy effectiveness after bariatric surgery. Ex vivo exposure increased primary NKT cell expression of antitumor cytokines, demonstrating direct activation of NKT cells by BCAAs. Together, the findings suggest that reinvigorating antitumor immunity may depend on bariatric surgery-associated microbially derived metabolites, namely BCAAs.
Keywords: Breast cancer; Cancer immunotherapy; Immunology; Microbiology; Obesity; Oncology.
Conflict of interest statement
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

