Vacuolar sterol β-glucosidase EGCrP2/Sgl1 deficiency in Cryptococcus neoformans: Dysfunctional autophagy and Mincle-dependent immune activation as targets of novel antifungal strategies
- PMID: 40273119
- PMCID: PMC12061408
- DOI: 10.1371/journal.ppat.1013089
Vacuolar sterol β-glucosidase EGCrP2/Sgl1 deficiency in Cryptococcus neoformans: Dysfunctional autophagy and Mincle-dependent immune activation as targets of novel antifungal strategies
Abstract
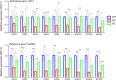
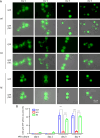
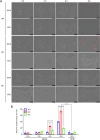
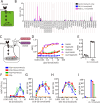
Cryptococcus neoformans (Cn) is a fungal pathogen responsible for cryptococcal meningitis, which accounts for 15% of AIDS-related deaths. Recent studies have shown that the absence of sterol β-glucosidase (EGCrP2, also known as Sgl1) in Cn significantly attenuates its virulence in a mouse infection model. However, the mechanisms underlying this virulence attenuation remain unclear. In this study, we observed a significant increase in dead cells after 3 days of culture of SGL1-deficient Cn (sgl1Δ, KO) at 37°C, compared with wild-type (WT) and SGL1-reconstituted Cn (sgl1Δ::SGL1, RE). qPCR analysis of WT, KO, and RE strains indicated that autophagy-related genes (ATGs) were significantly downregulated in KO strain. Atg8-dependent GFP translocation to the vacuole was significantly delayed in KO strain under starvation conditions. This autophagy dysfunction was identified as the primary cause of the increased cell death observed in KO strain under nitrogen starvation conditions at 37°C. EGCrP2/Sgl1 is predominantly localized in the vacuoles of Cn, and its deletion results in the accumulation of not only ergosterol β-glucoside (EG), as previously reported, but also acylated EGs (AEGs). AEGs were much more potent than EG in activating the C-type lectin receptor Mincle in mice, rats, and humans. AEGs were released from KO strain via extracellular vesicles (EVs). Chemically synthesized 18:1-EG and EVs derived from KO strain, but not WT or RE strains, enhanced cytokine production in murine and human dendritic cells. AEG-dependent cytokine production was markedly reduced in dendritic cells from Mincle-deficient mice, and the number of KO strain in lung tissue from Mincle-deficient mice was substantially higher than wild-type mice on day 3 after infection. Intranasal administration of acylated sitosterol β-glucoside increased Mincle expression and cytokine production and reduced the Cn burden in lung tissue of Cn-infected mice. These findings suggest that autophagy dysfunction in KO strain and the host innate immune response via the AEG-dependent Mincle activation are critical in reducing Cn virulence in mice.
Copyright: © 2025 Watanabe et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures












Similar articles
-
Limited Role of Mincle in the Host Defense against Infection with Cryptococcus deneoformans.Infect Immun. 2020 Oct 19;88(11):e00400-20. doi: 10.1128/IAI.00400-20. Print 2020 Oct 19. Infect Immun. 2020. PMID: 32868343 Free PMC article.
-
Identification of Aph1, a phosphate-regulated, secreted, and vacuolar acid phosphatase in Cryptococcus neoformans.mBio. 2014 Sep 16;5(5):e01649-14. doi: 10.1128/mBio.01649-14. mBio. 2014. PMID: 25227465 Free PMC article.
-
Sterylglucoside catabolism in Cryptococcus neoformans with endoglycoceramidase-related protein 2 (EGCrP2), the first steryl-β-glucosidase identified in fungi.J Biol Chem. 2015 Jan 9;290(2):1005-19. doi: 10.1074/jbc.M114.616300. Epub 2014 Oct 31. J Biol Chem. 2015. PMID: 25361768 Free PMC article.
-
Structure and inhibition of Cryptococcus neoformans sterylglucosidase to develop antifungal agents.Nat Commun. 2021 Oct 7;12(1):5885. doi: 10.1038/s41467-021-26163-5. Nat Commun. 2021. PMID: 34620873 Free PMC article.
-
Phenotypic switching and its implications for the pathogenesis of Cryptococcus neoformans.FEMS Yeast Res. 2006 Jun;6(4):480-8. doi: 10.1111/j.1567-1364.2006.00039.x. FEMS Yeast Res. 2006. PMID: 16696644 Free PMC article. Review.
References
-
- WHO fungal priority pathogens list to guide research, development and public health action [Internet]. n.d. [cited 2023 May 28]. Available from: https://www.who.int/publications/i/item/9789240060241
-
- Fisher MC, Denning DW. The WHO fungal priority pathogens list as a game-changer. Nature Reviews Microbiology. 2023. Feb 6 [cited 2023 Aug 30]; 21(4):211–2. https://www.nature.com/articles/s41579-023-00861-x - PMC - PubMed
-
- Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, et al.. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017. [cited 2024 Sep 10];17(8):873–81. https://pubmed.ncbi.nlm.nih.gov/28483415/ - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

