Distinct adipose progenitor cells emerging with age drive active adipogenesis
- PMID: 40273250
- PMCID: PMC12445215
- DOI: 10.1126/science.adj0430
Distinct adipose progenitor cells emerging with age drive active adipogenesis
Abstract
Starting at middle age, adults often suffer from visceral adiposity and associated adverse metabolic disorders. Lineage tracing in mice revealed that adipose progenitor cells (APCs) in visceral fat undergo extensive adipogenesis during middle age. Thus, despite the low turnover rate of adipocytes in young adults, adipogenesis is unlocked during middle age. Transplantations quantitatively showed that APCs in middle-aged mice exhibited high adipogenic capacity cell-autonomously. Single-cell RNA sequencing identified a distinct APC population, the committed preadipocyte, age-enriched (CP-A), emerging at this age. CP-As demonstrated elevated proliferation and adipogenesis activity. Pharmacological and genetic manipulations indicated that leukemia inhibitory factor receptor signaling was indispensable for CP-A adipogenesis and visceral fat expansion. These findings uncover a fundamental mechanism of age-dependent adipose remodeling, offering critical insights into age-related metabolic diseases.
Conflict of interest statement
Competing interests
Authors declare that they have no competing interests.
Figures
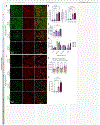
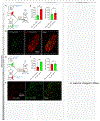





References
-
- Gallagher D et al. , Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr 72, 694–701 (2000). - PubMed
-
- Flegal KM, Carroll MD, Ogden CL, Curtin LR, Prevalence and trends in obesity among US adults, 1999-2008. Jama 303, 235–241 (2010). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

