The genetic landscape of sporadic adult-onset degenerative ataxia: a multi-modal genetic study of 377 consecutive patients from the longitudinal multi-centre SPORTAX cohort
- PMID: 40273470
- PMCID: PMC12051541
- DOI: 10.1016/j.ebiom.2025.105715
The genetic landscape of sporadic adult-onset degenerative ataxia: a multi-modal genetic study of 377 consecutive patients from the longitudinal multi-centre SPORTAX cohort
Abstract
Background: While most sporadic adult-onset neurodegenerative diseases have only a minor monogenic component, given several recently identified late adult-onset ataxia genes, the genetic burden may be substantial in sporadic adult-onset ataxias. We report systematic mapping of the genetic landscape of sporadic adult-onset ataxia in a well-characterised, multi-centre cohort, combining several multi-modal genetic screening techniques, plus longitudinal natural history data.
Methods: Systematic clinico-genetic analysis of a prospective longitudinal multi-centre cohort of 377 consecutive patients with sporadic adult-onset ataxia (SPORTAX cohort), including clinically defined sporadic adult-onset ataxia of unknown aetiology (SAOA) (n = 229) and 'clinically probable multiple system atrophy of cerebellar type' (MSA-Ccp) (n = 148). Combined GAA-FGF14 (SCA27B) and RFC1 repeat expansion screening with next-generation sequencing (NGS) was complemented by natural history and plasma neurofilament light chain analysis in key subgroups.
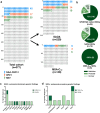
Findings: 85 out of 377 (22.5%) patients with sporadic adult-onset ataxia carried a pathogenic or likely pathogenic variant, thereof 67/229 (29.3%) patients with SAOA and 18/148 (12.2%) patients meeting the MSA-Ccp criteria. This included: 45/377 (11.9%) patients with GAA-FGF14≥250 repeat expansions (nine with MSA-Ccp), 17/377 (4.5%) patients with RFC1 repeat expansions (three with MSA-Ccp), and 24/377 (6.4%) patients with single nucleotide variants (SNVs) identified by NGS (six with MSA-Ccp). Five patients (1.3%) were found to have two relevant genetic variants simultaneously (dual diagnosis).
Interpretation: In this cohort of sporadic adult-onset ataxia, a cohort less likely to have a monogenic cause, a substantial burden of monogenic variants was identified, particularly GAA-FGF14 and RFC1 repeat expansions. This included a substantial share of patients meeting the MSA-Ccp criteria, suggesting a reduced specificity of this clinical diagnosis and potential co-occurrence of MSA-C plus a second, independent genetic condition. These findings have important implications for the genetic work-up and counselling of patients with sporadic ataxia, even when presenting with MSA-like features. With targeted treatments for genetic ataxias now on the horizon, these findings highlight their potential utility for these patients.
Funding: This work was supported by the Clinician Scientist programme "PRECISE.net" funded by the Else Kröner-Fresenius-Stiftung (to DM, AT, CW, OR, and MS), by the Deutsche Forschungsgemeinschaft (as part of the PROSPAX project), and by the Canadian Institutes of Health Research and the Fondation Groupe Monaco. Support was also provided by Humboldt Research Fellowship for Postdocs and the Hertie-Network of Excellence in Clinical Neuroscience and a Fellowship award from the Canadian Institutes of Health Research.
Keywords: Adult-onset ataxia; CANVAS; Disease trajectories; Genetic testing; Genomics; Multiple system atrophy; Prospective cohort; SCA27B; Sporadic ataxia.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Danique Beijer, Demet Önder, Carlo Wilke, Andreas Traschütz, Stefan Vielhaber, Thomas Klopstock, Florian Harmuth, Claudia Dufke, Bernard Brais, Olaf Rieß, Tobias B. Haack, Stephan Züchner, David Pellerin have no conflicts to report. David Mengel received funding from the Clinician Scientist program “PRECISE.net” funded by the Else Kröner-Fresenius-Stiftung, the Clinician Scientist program of the Medical Faculty Tübingen (459-0-0), Elite Program for Postdoctoral researchers of the Baden-Württemberg-Foundation (1.16101.21), Ministry of Science, Research and the Arts of the State of Baden-Württemberg and the ARSACS Foundation. Jennifer Faber received funding from the National Ataxia Foundation (NAF), was funded within the Advanced Clinician Scientist Programme (ACCENT, funding code 01EO2107). The ACCENT Program is funded by the German Federal Ministry of Education and Research (BMBF) and was funded as a PI within iBehave by the Ministry of Culture and Science of the State of North Rhine-Westphalia, Germany. JF was also reimbursed for education lecture expenses (diagnostic work-up and treatment of ataxia disorders) for practicing neurologists organised by continuing education companies; namely for organising a symposium for ASKLEPIOS Fachklinikum Stadtroda, and RG Gesellschaft für Information und Organisation mbH and has received consultancy honoraria as a participant of the strategic advisory board of VICO therapeutics. Dagmar Timmann is receiving funding from the German Research Foundation (DFG), European Union, Bernd Fink-Foundation and Mercur, all unrelated to the present manuscript. DT has received payment from University of Hamburg and support to attend meetings from German Research Foundation and Gordon Conference Cerebellum. Sylvia Boesch has received consulting fees from Biogen/Reata as well as honoraria from Biogen and Ipsen paid to the institution. SB has also participated in advisory boards for Vico Therapeutics and Biogen. SB has further received reimbursement of travel fees from Ipsen, Biogen/Reata and the Movement Disorders Society. Bart van de Warrenburg reports funding from Hersenstichting, ZonMw, The Netherlands organization for scientific Research, Christina Foundation, and consultancy honoraria from Biogen, Servier, VICO therapeutics, and Biohaven Pharmaceuticals, as well as royalties from BSL/Springer nature, and honoraria from Mov disord council Malaysia. BvdW has served on the Scientific advisory boards for Biogen and Vico Therapeutics. BvdW has further participated as chair or member of the board for MDS ataxia study group, Dutch ataxia guideline committee, Dutch ataxia patient society, Dutch HSP patient community and ERN-RND and received equipment from Brugling Fund, all unrelated to the present manuscript. Gabriella Silvestri has served as board member for aiViPS (Associazione italiana Vivere la Paraparesi Spastica) and of ARSACS odv. Christoph Kamm has received royalties from Elsevier (co-author book chapter), lecture honoraria from Ipsen and meeting/travel support from Ipsen and Merz. CK further participated in boards for Biogen, Ipsen and Roche, all unrelated to the submitted work. Iselin Marie Wedding reports no conflict of interest. Zofia Fleszar is supported by the MINT-Clinician Scientist program of the Medical Faculty Tübingen, funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—493,665,037. ZF has also received consultancy fees from Healthcare Manufaktur GmbH. Ludger Schöls has received funding from Servier and UCB as well as consulting fees from Vico Therapeutics and Vigil Neuroscience, and payment for expert testimony for Alexion and Novartis. Thomas Klockgether has received, in the last 24 months, consulting fees from Bristol-Myers Squibb (BMS), UCB and Arrowhead, all unrelated to the present manuscript. TK has also been involved in leadership of the Ataxia Global Initiative (AGI). Matthis Synofzik has received funding from the Else Kröner Fresenius Stiftung via a Clinician Scientist Programme Grant, as well as consultancy honoraria from Ionis Pharmaceuticals, UCB Pharmaceuticals, Prevail Pharmaceuticals, Orphazyme Pharmaceuticals, Servier Pharmaceuticals, Reata Pharmaceuticals, GenOrph, AviadoBio, Biohaven, Solaxa, Zevra, and Lilly, all unrelated to the present manuscript. The SPORTAX consortium is funded in part by institutional in-house funding from the German Center of Neurodegenerative Diseases (DZNE) for the contributing DZNE sites. Remaining contributions are from sites’ own funding.
Figures

References
-
- Schols L., Szymanski S., Peters S., et al. Genetic background of apparently idiopathic sporadic cerebellar ataxia. Hum Genet. 2000;107(2):132–137. - PubMed
-
- Abele M., Burk K., Schols L., et al. The aetiology of sporadic adult-onset ataxia. Brain. 2002;125(5):961–968. - PubMed
-
- Bogdan T., Wirth T., Iosif A., et al. Unravelling the etiology of sporadic late-onset cerebellar ataxia in a cohort of 205 patients: a prospective study. J Neurol. 2022;269(12):6354–6365. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

