Sex-dependent alterations in cardiac cytochrome P450-mediated arachidonic acid metabolism in pressure overload-induced cardiac hypertrophy in rats
- PMID: 40273825
- PMCID: PMC12163486
- DOI: 10.1016/j.dmd.2025.100077
Sex-dependent alterations in cardiac cytochrome P450-mediated arachidonic acid metabolism in pressure overload-induced cardiac hypertrophy in rats
Abstract
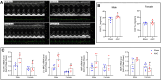
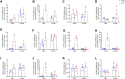
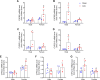
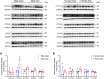
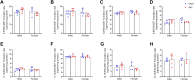
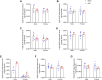
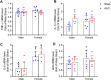
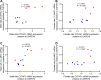
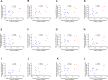
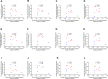
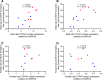
Cardiac hypertrophy is a risk factor for heart failure and is usually less common in young women than in men. Cytochrome P450 (CYP) enzymes in the heart metabolize arachidonic acid into hydroxyeicosatetraenoic acids (HETEs), which generally have hypertrophic effects, and epoxyeicosatrienoic acids, which have cardioprotective effects. In this study, we aimed to investigate sex-specific differences in cardiac hypertrophy and cardiac CYP, HETE, and epoxyeicosatrienoic acid levels in response to pressure overload. Adult male and female Sprague-Dawley rats were subject to sham or abdominal aortic constriction (AAC) surgeries. Five weeks postsurgery, cardiac function was assessed by echocardiography. The mRNA and protein levels of hypertrophic markers and CYP enzymes were measured by real-time polymerase chain reaction and Western blot. Heart tissue HETE levels and microsomal formation of HETEs and epoxyeicosatrienoic acids were measured by liquid chromatography-tandem mass spectrometry. Our results show significant sex-specific differences in AAC-induced cardiac hypertrophy. Echocardiography and ventricular wall measurements showed more hypertrophy in male rats. Some hypertrophic markers were significantly upregulated only in male AAC rats and were significantly higher in the hearts of male rats compared to female AAC rats. Different CYP hydroxylases such as CYP1B1, CYP4A, and CYP4F and epoxygenases such as CYP2C and CYP2J10 were significantly upregulated in the hearts of male AAC rats only. The heart level of 12(R)-HETE and the microsomal formation of several HETEs were also significantly increased only in male rats. In conclusion, male rats developed stronger AAC-induced cardiac hypertrophy compared to female rats, which was accompanied by a significant increase in cardiac CYP enzymes and HETEs. SIGNIFICANCE STATEMENT: Previous studies demonstrated that male rats experience more severe cardiac hypertrophy compared to female rats. To our knowledge, this research is the first to investigate and compare the expression of cytochrome P450 enzymes and arachidonic acid metabolites in male and female rat hearts following pressure overload-induced hypertrophy. This study highlights significant sex-specific differences in cytochrome P450-mediated metabolism during hypertrophy, providing valuable insights into the molecular mechanisms underlying these responses and identifying potential targets for sex-specific therapies in cardiac diseases.
Keywords: Arachidonic acid; Cardiac hypertrophy; Cytochrome P450; Epoxyeicosatrienoic acids; Hydroxyeicosatetraenoic acids.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflicts of interest.
Figures

References
-
- Althurwi H.N., Maayah Z.H., Elshenawy O.H., El-Kadi A.O. Early changes in cytochrome P450s and their associated arachidonic acid metabolites play a crucial role in the initiation of cardiac hypertrophy induced by isoproterenol. Drug Metab Dispos. 2015;43:1254–1266. - PubMed
-
- Berkowicz P., Kij A., Walczak M., Chlopicki S. Eicosanoid profiling in effluent of isolated perfused heart of Tgαq∗44 mice with advanced heart failure. J Physiol Pharmacol. 2019;70:135–142. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

