Gut microbiota derived indole-3-acetic acid ameliorates precancerous inflammatory intestinal milieu to inhibit tumorigenesis through IL-35
- PMID: 40274281
- PMCID: PMC12020765
- DOI: 10.1136/jitc-2024-011155
Gut microbiota derived indole-3-acetic acid ameliorates precancerous inflammatory intestinal milieu to inhibit tumorigenesis through IL-35
Abstract
Background: Gut microbiota can significantly alter the risk or progression of cancer by maintaining gut immune system homeostasis. However, the exact mechanism by which the gut microbiota and its metabolites influence colorectal tumorigenesis is unclear.
Methods: The roles of tryptophan metabolite indole-3-acetic acid (IAA) in inflammation and tumor development were investigated in dextran sodium sulfate (DSS) and azoxymethane (AOM)-DSS mouse models with or without IAA supplementation and with or without Lactobacillus reuteri-produced IAA. Pregnane X receptor (PXR) knockout (KO) mice and aryl hydrocarbon receptor KO mice were used to explore the mechanism by which IAA regulates interleukin (IL)-35 expression. IL-35+ immune cells were stimulated in vitro and analyzed by flow cytometry. Additionally, metabolites were analyzed by liquid chromatography-mass spectrometry.
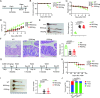
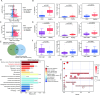
Results: We found that IAA, a metabolite of tryptophan produced in the gut by L. reuteri, can inhibit the development of colitis by inducing IL-35 expression in immunosuppressant cells. HuREG3αIECtg mice had high levels of intestinal microbiota-derived IAA, and these mice were resistant to AOM-DSS-induced cancer. Patients with colorectal cancer also had low peripheral blood levels of IAA. Further studies revealed that IAA-producing L. reuteri alleviated colitis symptoms and inhibited colon tumors by inducing macrophages, T cells, and B cells to produce IL-35. Finally, PXR KO completely abolished the effects of IAA on immune cells.
Conclusion: We demonstrate that gut microbiota-derived IAA can improve the precancerous colon inflammatory environment through IL-35, thereby inhibiting tumorigenesis, suggesting that IAA may be a preventive factor for colitis-related cancers.
Keywords: Colorectal Cancer; Immune modulatory; Immunosuppression; Macrophage.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures





Similar articles
-
Gut microbiota metabolite indole-3-acetic acid maintains intestinal epithelial homeostasis through mucin sulfation.Gut Microbes. 2024 Jan-Dec;16(1):2377576. doi: 10.1080/19490976.2024.2377576. Epub 2024 Jul 27. Gut Microbes. 2024. PMID: 39068517 Free PMC article.
-
Bifidobacterium breve-derived indole-3-lactic acid ameliorates colitis-associated tumorigenesis by directing the differentiation of immature colonic macrophages.Theranostics. 2024 Apr 22;14(7):2719-2735. doi: 10.7150/thno.92350. eCollection 2024. Theranostics. 2024. PMID: 38773969 Free PMC article.
-
Indole-3-acetic acid ameliorates dextran sulfate sodium-induced colitis via the ERK signaling pathway.Arch Pharm Res. 2024 Mar;47(3):288-299. doi: 10.1007/s12272-024-01488-z. Epub 2024 Mar 15. Arch Pharm Res. 2024. PMID: 38489148
-
Gut microbiota - indole-3-acetic acid axis in cancer: dual functions, mechanistic insights, and therapeutic potential.Microbiol Res. 2025 Nov;300:128293. doi: 10.1016/j.micres.2025.128293. Epub 2025 Jul 28. Microbiol Res. 2025. PMID: 40752133 Review.
-
Multifaceted Role of Microbiota-Derived Indole-3-Acetic Acid in Human Diseases and Its Potential Clinical Application.FASEB J. 2025 Jun 15;39(11):e70574. doi: 10.1096/fj.202500295R. FASEB J. 2025. PMID: 40415505 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
