Novel humanized CD19-CAR-T (Now talicabtagene autoleucel, Tali-cel™) cells in relapsed/ refractory pediatric B-acute lymphoblastic leukemia- an open-label single-arm phase-I/Ib study
- PMID: 40274761
- PMCID: PMC12022059
- DOI: 10.1038/s41408-025-01279-9
Novel humanized CD19-CAR-T (Now talicabtagene autoleucel, Tali-cel™) cells in relapsed/ refractory pediatric B-acute lymphoblastic leukemia- an open-label single-arm phase-I/Ib study
Erratum in
-
Correction: Novel humanized CD19-CAR-T (Now talicabtagene autoleucel, Tali-cel™) cells in relapsed/ refractory pediatric B-acute lymphoblastic leukemia- an open-label single-arm phase-I/Ib study.Blood Cancer J. 2025 May 20;15(1):98. doi: 10.1038/s41408-025-01308-7. Blood Cancer J. 2025. PMID: 40393961 Free PMC article. No abstract available.
Abstract
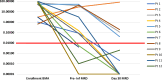
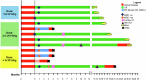
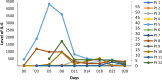
Chimeric Antigen Receptor-T (CAR-T) cell therapy is effective for relapsed/refractory B-acute lymphoblastic leukemia (r/r B-ALL) but is not universally available. We developed a novel humanized CD19-directed CAR-T (HCAR19) approved for Phase 1/1b/2 trials. Patients aged 3-25 years were enrolled with r/r B-ALL and ineligible for allogeneic stem cell transplant. Lymphodepletion utilized standard-dose fludarabine and cyclophosphamide. A 3 + 3 design testing 3 dose-ranges was used to determine Phase-2 Dose (P2D): Dose-A, 1 × 106 HCAR19 cells/kg, Dose-B, 3-5 × 106/kg, and Dose-C, 10-15 × 106/kg. Primary endpoint was overall response rate (ORR) at day-30 on bone-marrow flow-cytometry. From May-2021 to September-2023 12 patients [median age-14 (range: 5-24) years] were enrolled with median bone marrow blasts 19.5% at screening. Cytokine release syndrome occurred in 10 (83%) patients, predominantly Grades 1-2, and Grade-2 immune-cell associated neurotoxicity (ICANS) in 1. All patients had Grade-3 cytopenia. ORR was 91.7% (11/12), complete response (CR) in 8 (66.7%) and partial response in 3 (25%). Seven of 8 CRs were at Dose-levels B and C, all of which were sustained till 12 months follow-up. Patients who received dose levels below 3 × 106/kg, or did not achieve CR, had early loss of response or rapid progression. HCAR19 demonstrated safety, manageable toxicity, and durable remissions. and P2D was determined as 5-10 × 106 HCAR19-cells/kg. CLINICAL TRIAL REGISTRATION: The study is registered in the Clinical Trials Registry- India (CTRI/2021/05/033348 and CTRI/2023/03/050689).
© 2025. The Author(s).
Conflict of interest statement
Competing interests: GN serves as a scientific advisor/board member in ImmunoACT. HJ has received research and/or clinical trial support from ImmunoACT, Zydus, and Intas Pharmaceuticals. RP, AK, SS, and AF are equity holders in a private company, have memberships on the entity’s Board of Directors or advisory committees, hold patents and royalties with ImmunoACT, and are currently employed there. JT serves as a consultant for ImmunoACT. NNS is a consultant and/or advisory board member for ImmunoACT, VOR, Cargo, and Lentigen. The remaining authors declare no conflict of interest. Ethics approval: The prospective study was approved by the institutional review boards and ethical committee clearance was obtained (ECR/149/Inst/MH/2023 and IITB-IEC/2018/023). Informed consent: Informed consent was obtained from the caretakers of the patients in our study.
Figures


References
-
- Hunger SP, Mullighan CG. Acute Lymphoblastic Leukemia in Children. N Engl J Med. 2015;373:1541–52. - PubMed
-
- Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166. - PubMed
-
- Gaynon PS. Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol. 2005;131:579–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

