SOX5 inhibition overcomes PARP inhibitor resistance in BRCA-mutated breast and ovarian cancer
- PMID: 40274769
- PMCID: PMC12022250
- DOI: 10.1038/s41419-025-07660-7
SOX5 inhibition overcomes PARP inhibitor resistance in BRCA-mutated breast and ovarian cancer
Abstract
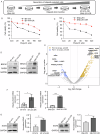
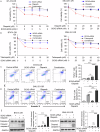
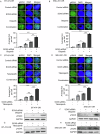
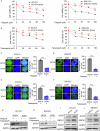
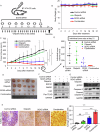
Poly (ADP-ribose) polymerase (PARP) inhibitors are effective in cells with homologous recombination (HR) deficiency, including BRCA1/2 mutation. However, PARP inhibitors remain a therapeutic challenge in breast and ovarian cancer due to inevitably acquired resistance in most cases. Therefore, strategies to overcome PARP inhibitor resistance are unmet clinical need. SRY-box transcription factor 5 (SOX5) plays a crucial role in development of various cancers but the role of SOX5 in PARP inhibitor resistance is poorly understood. This study identified SOX5 as a potential biomarker associated with PARP inhibitor resistance and addressed potential treatment strategies to overcome PARP inhibitor resistance using the olaparib-resistant preclinical model. We observed that SOX5 was significantly upregulated in olaparib-resistant cells and contributed to PARP inhibitor resistance by upregulating DNA repair pathway genes. Ectopic SOX5 overexpression contributed to PARP inhibitor resistance by suppressing DNA double-strand breaks (DSBs) in BRCA-mutated breast and ovarian cancer. SOX5 small interfering RNA combined with olaparib sensitized olaparib-resistant cells and suppressed the growth of olaparib-resistant xenografts in mice via increased DSBs represented by ɣH2AX formation. Mechanistically, SOX5 directly interacted with yes-associated protein 1 (YAP1) and promoted its nuclear translocation by suppressing the Hippo pathway. YAP1, in association with TEA domain family members (TEAD), upregulated HR-related gene expression and conferred PARP inhibitor resistance. Furthermore, the clinical relevance of SOX5 as a therapeutic target was supported by a significant association between SOX5 overexpression and poor prognosis in ovarian cancer on public mRNA microarray data sets. Therefore, we propose SOX5 as a promising therapeutic target for overcoming PARP inhibitor resistance in BRCA1/2-mutated breast and ovarian cancer.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The corresponding author received research funds from several pharmaceutical companies, including ImmunoMet Therapeutics, Hanmi Pharmaceutical Co., and Celltrion. The other authors declare no conflict of interest. Ethics: All animal procedures were performed according to the approved protocol by the Institutional Animal Care and Use Committee (IACUC) of CHA University (IACUC230054).
Figures

Similar articles
-
ALDH1A1 Contributes to PARP Inhibitor Resistance via Enhancing DNA Repair in BRCA2-/- Ovarian Cancer Cells.Mol Cancer Ther. 2020 Jan;19(1):199-210. doi: 10.1158/1535-7163.MCT-19-0242. Epub 2019 Sep 18. Mol Cancer Ther. 2020. PMID: 31534014 Free PMC article.
-
Molecular mechanisms restoring olaparib efficacy through ATR/CHK1 pathway inhibition in olaparib-resistant BRCA1/2MUT ovarian cancer models.Biochim Biophys Acta Mol Basis Dis. 2025 Feb;1871(2):167574. doi: 10.1016/j.bbadis.2024.167574. Epub 2024 Nov 16. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 39557132
-
PARP inhibitor resistance and TP53 mutations in patients treated with olaparib for BRCA-mutated cancer: Four case reports.Mol Med Rep. 2021 Jan;23(1):75. doi: 10.3892/mmr.2020.11713. Epub 2020 Nov 25. Mol Med Rep. 2021. PMID: 33236159
-
Advances in PARP inhibitors for the treatment of breast cancer.Expert Opin Pharmacother. 2015;16(18):2751-8. doi: 10.1517/14656566.2015.1100168. Epub 2015 Oct 20. Expert Opin Pharmacother. 2015. PMID: 26485111 Review.
-
Advances in the Treatment of Ovarian Cancer Using PARP Inhibitors and the Underlying Mechanism of Resistance.Curr Drug Targets. 2020;21(2):167-178. doi: 10.2174/1389450120666190925123507. Curr Drug Targets. 2020. PMID: 31553293 Review.
Cited by
-
89Zr-Radiolabelling of p-NCS-Bz-DFO-Anti-HER2 Affibody Immunoconjugate: Characterization and Assessment of In Vitro Potential in HER2-Positive Breast Cancer Imaging.Pharmaceutics. 2025 Jun 4;17(6):739. doi: 10.3390/pharmaceutics17060739. Pharmaceutics. 2025. PMID: 40574051 Free PMC article.
-
Liquid biopsy in breast cancer: Redefining precision medicine.J Liq Biopsy. 2025 Jul 16;9:100312. doi: 10.1016/j.jlb.2025.100312. eCollection 2025 Sep. J Liq Biopsy. 2025. PMID: 40740670 Free PMC article. Review.
References
-
- O’Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015;60:547–60. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

