Comparing neural responses to cutaneous heat and pressure pain in healthy participants
- PMID: 40274927
- PMCID: PMC12022288
- DOI: 10.1038/s41598-025-99247-7
Comparing neural responses to cutaneous heat and pressure pain in healthy participants
Abstract
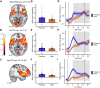
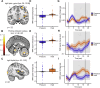
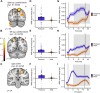
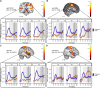
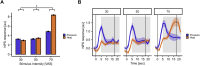
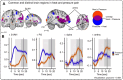
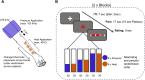
Even though acute pain comes in many different shapes and forms, a lot of experimental pain studies predominantly employ cutaneous heat pain. This makes a comparison between different pain types and the link between findings from these experimental studies to clinical pain difficult. To bridge this gap, we investigated both cuff pressure pain and cutaneous heat pain using a within-subject design in combination with functional magnetic resonance imaging (fMRI). Noxious stimuli were applied with a 17-s duration at three different intensities above the pain threshold using a thermode and a computer-controlled cuff pressure device. Both pain modalities led to contralateral activation in the anterior insula and parietal operculum. Heat pain showed greater activation in the precentral gyrus, pontine reticular nucleus, and dorsal posterior insula, whilst pressure pain showed greater activation in the primary somatosensory cortex and bilateral superior parietal lobules. Most importantly, the time course of the fMRI signal changes differed between modalities, with pressure pain peaking in the first stimulus half, whereas heat pain led to a prolonged and increasing response across the stimulus duration with a peak in the second stimulus half. Our findings suggest that pressure and heat pain lead to common as well as different (temporal) activation patterns in key pain processing regions.
Keywords: Cuff pressure; Cutaneous heat; Neuroimaging; Nociception; Pain; fMRI.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Multiple somatotopic representations of heat and mechanical pain in the operculo-insular cortex: a high-resolution fMRI study.J Neurophysiol. 2010 Nov;104(5):2863-72. doi: 10.1152/jn.00253.2010. Epub 2010 Aug 25. J Neurophysiol. 2010. PMID: 20739597 Free PMC article.
-
Differentiating noxious- and innocuous-related activation of human somatosensory cortices using temporal analysis of fMRI.J Neurophysiol. 2002 Jul;88(1):464-74. doi: 10.1152/jn.2002.88.1.464. J Neurophysiol. 2002. PMID: 12091568
-
Temporal summation of heat pain in humans: Evidence supporting thalamocortical modulation.Pain. 2010 Jul;150(1):93-102. doi: 10.1016/j.pain.2010.04.001. Epub 2010 May 21. Pain. 2010. PMID: 20494516 Free PMC article.
-
Functional imaging of brain responses to pain. A review and meta-analysis (2000).Neurophysiol Clin. 2000 Oct;30(5):263-88. doi: 10.1016/s0987-7053(00)00227-6. Neurophysiol Clin. 2000. PMID: 11126640 Review.
-
Central mechanisms of pain perception.Suppl Clin Neurophysiol. 2004;57:39-49. doi: 10.1016/s1567-424x(09)70341-1. Suppl Clin Neurophysiol. 2004. PMID: 16106604 Review.
References
-
- Capra, N. F. & Ro, J. Y. Human and animal experimental models of acute and chronic muscle pain: Intramuscular algesic injection. Pain110, 3 (2004). - PubMed
-
- Owen, D. G., Clarke, C. F., Ganapathy, S., Prato, F. S. & St. Lawrence, K. S. Using perfusion MRI to measure the dynamic changes in neural activation associated with tonic muscular pain. Pain148, 375 (2010). - PubMed
-
- Svensson, P., Minoshima, S., Beydoun, A., Morrow, T. J. & Casey, K. L. Cerebral processing of acute skin and muscle pain in humans. J. Neurophysiol.78, 450–460 (1997). - PubMed
-
- Thunberg, J. et al. Brain processing of tonic muscle pain induced by infusion of hypertonic saline. Eur. J. Pain9, 185–194 (2005). - PubMed
-
- Graven-Nielsen, T., Arendt-Nielsen, L., Svensson, P. & Jensen, T. S. Experimental muscle pain: A quantitative study of local and referred pain in humans following injection of hypertonic saline. J. Musculoskelet. Pain5, 49–69 (1997). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

