Myeloid neoplasms after CD19-directed CAR T cells therapy in long-term B-cell lymphoma responders, a rising risk over time?
- PMID: 40275069
- PMCID: PMC12208870
- DOI: 10.1038/s41375-025-02605-7
Myeloid neoplasms after CD19-directed CAR T cells therapy in long-term B-cell lymphoma responders, a rising risk over time?
Abstract
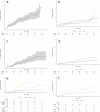
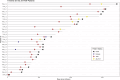
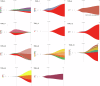
Therapy-related myeloid neoplasms (t-MN), including myelodysplastic neoplasms (t-MDS) and acute myeloid leukemia (t-AML), have emerged as significant late complications after CAR T cell therapy. We retrospectively analyzed 539 patients with B cell lymphoma treated with CD19 directed CAR T cell therapy across four French centers. Cumulative incidences of t-MN was estimated with relapse or death treated as competing risk. Univariate and propensity score matching (PSM) analyses were conducted to assess risk factors with age and the number of prior treatments as covariates. After a median follow-up of 25 months, the cumulative incidence of t-MN was 4.5% at 2 years. T-MN occurred predominantly as t-MDS (62%) and t-AML (38%) with high cytogenetic risk. Median overall survival after t-MN diagnosis was 4.5 months. In univariate analysis, older age (p < 0.01), higher MCV (p < 0.01), and higher ICANS grade (p = 0.04) were associated with increased risk of t-MN. After PSM, MCV and ICANS grade remained significant risk factors. CAR T cell products with CD28 co-stimulatory domains trended towards higher t-MN risk (p = 0.09). NGS analysis showed that 85.7% of t-MN had pre-existing mutations, most commonly TP53. This study highlights t-MN as a severe late complication of CAR T cell therapy. MCV and ICANS grade were identified as key risk factors.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: NGa, RT, MS, GD, NGo, LF, PFG, ND, BP, JD, SH, PS, HG, MH, PS have no COI. DB declares honorariua for Kite/Gilead and Novartis, EF declares consultancy for Astra Zeneca, Beigene, Janssen and honoraria for Astra Zeneca, Beigene, Janssen, Cilag, Abbvie, Gilead, VS declares consultancy for Kite/ Gilead, Beigene, BMS, Janssen and other from Janssen, EB declares consultancy, honoraria, and membership on an entity’s Board of Directors or advisory committees from AbbVie, Roche, and Takeda; research funding from Amgen; honoraria and membership on an entity’s Board of Directors or advisory committees from BeiGene and Incyte; honoraria, other personal fees, and research funding from Bristol Myers Squibb; consultancy and honoraria from Janssen; honoraria and other personal fees from Novartis and Pfizer; honoraria from ADC Therapeutics; and consultancy, honoraria, and other personal fees from Kite, a Gilead Company, FM declares consultancy, honoraria, and membership on an entity’s Board of Directors or advisory committees from Kite/Gilead Sciences, Bristol Myers Squibb, AbbVie, Epizyme, AstraZeneca, Novartis, and Genmab; consultancy and membership on an entity’s Board of Directors or advisory committees from Servier; consultancy, honoraria, and other payments for expert testimony and scientific lectures from Roche/Genentech; and honoraria from Chugai and Eisai, IYA declares honoraria from KITE, BMS, Novartis, Miltenyi Biomedecine.
Figures

References
-
- Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380:45–56. - PubMed
-
- Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52. - PubMed
-
- Fowler NH, Dickinson M, Dreyling M, Martinez-Lopez J, Kolstad A, Butler J, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2022;28:325–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

