ATM aberrations in chronic lymphocytic leukemia: del(11q) rather than ATM mutations is an adverse-prognostic biomarker
- PMID: 40275070
- PMCID: PMC12208880
- DOI: 10.1038/s41375-025-02615-5
ATM aberrations in chronic lymphocytic leukemia: del(11q) rather than ATM mutations is an adverse-prognostic biomarker
Abstract
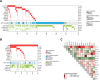
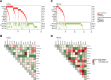
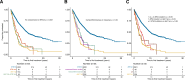
Despite the well-established adverse impact of del(11q) in chronic lymphocytic leukemia (CLL), the prognostic significance of somatic ATM mutations remains uncertain. We evaluated the effects of ATM aberrations (del(11q) and/or ATM mutations) on time-to-first-treatment (TTFT) in 3631 untreated patients with CLL, in the context of IGHV gene mutational status and mutations in nine CLL-related genes. ATM mutations were present in 246 cases (6.8%), frequently co-occurring with del(11q) (112/246 cases, 45.5%). ATM-mutated patients displayed a different spectrum of genetic abnormalities when comparing IGHV-mutated (M-CLL) and unmutated (U-CLL) cases: M-CLL was enriched for SF3B1 and NFKBIE mutations, whereas U-CLL showed mutual exclusivity with trisomy 12 and TP53 mutations. Isolated ATM mutations were rare, affecting 1.2% of Binet A patients and <1% of M-CLL cases. While univariable analysis revealed shorter TTFT for Binet A patients with any ATM aberration compared to ATM-wildtype, multivariable analysis identified only del(11q), trisomy 12, SF3B1, and EGR2 mutations as independent prognosticators of shorter TTFT among Binet A patients and within M-CLL and U-CLL subgroups. These findings highlight del(11q), and not ATM mutations, as a key biomarker of increased risk of early progression and need for therapy, particularly in otherwise indolent M-CLL, providing insights into risk-stratification and therapeutic decision-making.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: PG: Honoraria/advisory board: AbbVie, Acerta/AstraZeneca, Adaptive, ArQule/MSD, BeiGene, CelGene/Juno, Gilead, Janssen, Loxo/Lilly, Sunesis. Research funding: AbbVie, Gilead, Janssen, Novartis, Sunesis; LS: advisory board AbbVie, AstraZeneca, Janssen; RR: honoraria/advisory board: AbbVie, AstraZeneca, Illumina, Janssen, Lilly and Roche; KS: honoraria/advisory board: AbbVie, Acerta/AstraZeneca, Gilead, Janssen. Research funding: AbbVie, Gilead, Janssen; PB: honoraria from Abbvie, Gilead and Janssen. Research funding from Gilead; GG: Advisory Board/Speaker’s bureau: Abbvie, AstraZeneca, Beigene, Hikma, Incyte, Johnson & Johnson, Lilly; LB: honoraria/advisory board: Abbvie, Amgen, Astellas, BMS/Celgene, Daiichi Sankyo, Gilead, Hexal, Janssen, Jazz Pharmaceuticals, Menarini, Novartis, Pfizer, Sanofi, Seattle Genetics. Research funding: Bayer, Jazz Pharmaceuticals; GMR: honoraria from Abbvie, AstraZeneca, Gilead and Janssen. Research funding from Gilead; CB: Honoraria/advisory board: AstraZeneca and Eli Lilly. CUN received research grants and/or honoraria from Abbvie, AstraZeneca, Janssen, Genmab, Beigene, Octapharma, MSD, Lilly, Synamics, CSL Behring, Takeda, Nofo Nordisk Foundation. The other authors declare no competing financial interests
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

