Evaluation of the antiproliferative, cytotoxic and phytochemical properties of Zimbabwean medicinal plants used in cancer treatment
- PMID: 40275320
- PMCID: PMC12023620
- DOI: 10.1186/s12906-025-04883-1
Evaluation of the antiproliferative, cytotoxic and phytochemical properties of Zimbabwean medicinal plants used in cancer treatment
Abstract
Background: Cancer cases have been on the rise globally and several treatment strategies have been developed but mortality rates remain high. Zimbabwe, like many other countries, has also experienced a surge in cancer cases. In Zimbabwe, medicinal plants have been widely used to treat cancer for centuries. However, there has been limited research on the effectiveness, safety, and chemical composition of these plants. The current study assessed antiproliferative, cytotoxic and phytochemical properties of selected Zimbabwean medicinal plants.
Method: Cytotoxic activity of Agelenthus pungu, Carissa edulis, Dombeya rotundifolia, Flacourtia indica, Lannea discolor, Leonotis ocymifolia, Leucas martinicensis, Plicosepalus kalachariensis, Pseudolachnostylis maproneifolia, Solanum incanum, Strychnos cocculoides, Strychnos spinosa and Viscum verrucosum extracts were evaluated on normal murine peritoneal cells and sheep erythrocytes while antiproliferative activity was assessed on Jurkat T and HL60 cell lines. Cell viability was determined using the trypan blue exclusion and sulforhodamine B assay. Additionally, the effect of reduced glutathione on cytotoxic extracts was examined. The phytochemicals of the methanolic extracts were qualitatively determined using standard methods.
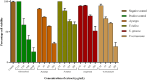
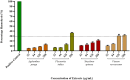
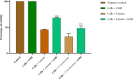
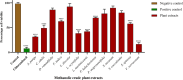
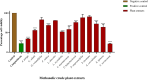
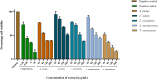
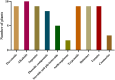
Results: Agelenthus pungu, Carissa edulis, Flacourtia indica, Strychnos cocculoides, Strychnos spinosa and Viscum verrucosum were cytotoxic to normal murine peritoneal cells. Flacourtia indica and Viscum verruscosum caused haemolysis of sheep erythrocytes at a concentration of 250 µg/mL for both plant extracts and 125 µg/mL for Viscum verrucosum. Cell viability increased on addition of 25 µg/mL of reduced glutathione to the extracts considered the most cytotoxic extracts, Agelenthus pungu and Viscum verrucosum. Agelenthus pungu, Carissa edulis, Leonotis ocymifolia, Leucas martinicensis and Viscum verrucosum significantly inhibited Jurkat T and HL60 cell proliferation. Viscum verrucosum was the most potent with the lowest half-maximum inhibitory concentration (IC50) values of 33 and 34 µg/mL on Jurkat T and HL60 cell lines respectively. The most dominant phytochemical classes were alkaloids, flavonoids and saponins.
Conclusion: This study demonstrates that Agelenthus pungu, Carissa edulis, Leonotis ocymifolia, Leucas martinicensis and Viscum verrucosum have antiproliferative activity against Jurkat T and HL60 cell lines. Viscum verrucosum was the most potent. These findings emphasise the importance of medicinal plants as well as their potential use as sources of novel compounds in anticancer drug discovery.
Keywords: Viscum verrucosum; Antiproliferative; Cancer; Cytotoxicity; Medicinal plants; Phytochemistry; Zimbabwe.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Faculty Higher Degrees Committee during its 318th meeting in November 2017 at the National University of Science and Technology (NUST), Zimbabwe. This approval was granted in the form of a written project proposal discussed in the board meeting. The NUST Institutional Review Board also approved the study, assigning it approval number NUST/IRB/2022/48. Permission to collect plant species was obtained from the relevant Rural District Councils and Community Leaders. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Viscum articulatum Burm. f. aqueous extract exerts antiproliferative effect and induces cell cycle arrest and apoptosis in leukemia cells.J Ethnopharmacol. 2018 Jun 12;219:91-102. doi: 10.1016/j.jep.2018.03.005. Epub 2018 Mar 16. J Ethnopharmacol. 2018. PMID: 29555410
-
In vitro anthelmintic activity of crude extracts of five medicinal plants against egg-hatching and larval development of Haemonchus contortus.J Ethnopharmacol. 2011 Sep 1;137(1):108-13. doi: 10.1016/j.jep.2011.04.063. Epub 2011 May 6. J Ethnopharmacol. 2011. PMID: 21605649
-
Phytochemical Fingerprinting and Activity of Extracts from the Leaves of Dolichos kilimandscharicus (Fabaceae) on Jurkat-T Cells.Biomed Res Int. 2020 Oct 5;2020:1263702. doi: 10.1155/2020/1263702. eCollection 2020. Biomed Res Int. 2020. PMID: 33083448 Free PMC article.
-
Current Status and Future Perspective for Research on Medicinal Plants with Anticancerous Activity and Minimum Cytotoxic Value.Curr Drug Targets. 2019;20(12):1227-1243. doi: 10.2174/1389450120666190429120314. Curr Drug Targets. 2019. PMID: 31486747 Review.
-
Anticancer medicinal plants used by Moroccan people: Ethnobotanical, preclinical, phytochemical and clinical evidence.J Ethnopharmacol. 2021 Feb 10;266:113435. doi: 10.1016/j.jep.2020.113435. Epub 2020 Oct 3. J Ethnopharmacol. 2021. PMID: 33022340 Review.
References
-
- Hofmarcher T, Manzano García A, Wilking N & Lindgren P. The Disease Burden and Economic Burden of Cancer in 9 Countries in the Middle East and Africa. Value Health Reg. 2023;37:81–87. - PubMed
-
- Ferlay J, et al. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021. 10.1002/ijc.33588. - PubMed
-
- Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Parkin DM, et al. Changes in the Incidence of Cancer in Bulawayo, Zimbabwe over a 50-Year Period. Cancer Epidemiol Biomarkers Prev. 2021;30:867–73. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

