The Cost-Effectiveness of a Multi-Target Stool DNA-Based Screening (COLOTECT), FIT, Colonoscopy and No Screening for Colorectal Cancer
- PMID: 40275466
- PMCID: PMC12021674
- DOI: 10.1002/cnr2.70176
The Cost-Effectiveness of a Multi-Target Stool DNA-Based Screening (COLOTECT), FIT, Colonoscopy and No Screening for Colorectal Cancer
Abstract
Background: Around 1.9 million new cases and 1 million deaths worldwide were attributed to colorectal cancer (CRC) in 2020.
Aims: The aims of this study are to assess the cost-effectiveness of a multi-target stool DNA-based screening strategy, COLOTECT, compared to faecal immunochemical tests (FIT), colonoscopy, and no screening in the Asian population to inform more choices for policymakers in colorectal cancer screening.
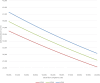
Method and results: We assume that 100,000 persons aged 50 undergo annual FIT, annual COLOTECT multi-target testing, or colonoscopies every 10 years until age 75. The data used in this study was retrieved from different sources including the Hong Kong Cancer Registry and previously published studies on the population aged 50 to 75 years old between 2010 and 2023. This study accessed the most cost-effective screening strategy available. If a positive result of FIT or COLOTECT were observed, the participants would undergo a colonoscopy. The participants who used the colonoscopy as the main screening method conducted colonoscopies every 3 years. The Markov models were utilized to compare the outcomes from different strategies including life-years saved, years of life lost, and incremental cost-effectiveness ratio (primary outcome). The highest ICER was observed in colonoscopy (USD 160808), followed by FIT (USD 108952), and COLOTECT (USD 82206). A higher detection rate of CRC (COLOTECT: 39.3% vs. FIT: 4.5%), more CRC cases prevented (1272 vs. 146), and life-years saved (2295 vs. 337) were observed in the COLOTECT strategy than in FIT. Additionally, a lower total cost per life-year saved of COLOTECT (USD 180097) was observed than colonoscopy (USD 238356), which identified the more affordable and cost-saving COLOTECT strategy.
Conclusion: This study highlighted the better performance of COLOTECT than FIT in detecting CRC. Additionally, given its lower cost and higher acceptance, the COLOTECT strategy might be more cost-effective than colonoscopy for massive CRC screening.
Keywords: colorectal cancer; cost‐effectiveness; multi‐target stool DNA; non‐invasive biomarker; screening.
© 2025 The Author(s). Cancer Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Sung H., Ferlay J., Siegel R. L., et al., “Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries,” CA: A Cancer Journal for Clinicians 71, no. 3 (2021): 209–249. - PubMed
-
- Hong Kong Cancer Registry , “Overview of Hong Kong Cancer Statistics of 20222024,”, https://www3.ha.org.hk/cancereg, accessed 6 February 2025.
-
- Kronborg O., Fenger C., Olsen J., Jørgensen O. D., and Søndergaard O., “Randomised Study of Screening for Colorectal Cancer With Faecal‐Occult‐Blood Test,” Lancet 348, no. 9040 (1996): 1467–1471. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

