Myo-Guide: A Machine Learning-Based Web Application for Neuromuscular Disease Diagnosis With MRI
- PMID: 40275674
- PMCID: PMC12022233
- DOI: 10.1002/jcsm.13815
Myo-Guide: A Machine Learning-Based Web Application for Neuromuscular Disease Diagnosis With MRI
Abstract
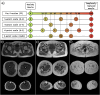
Background: Neuromuscular diseases (NMDs) are rare disorders characterized by progressive muscle fibre loss, leading to replacement by fibrotic and fatty tissue, muscle weakness and disability. Early diagnosis is critical for therapeutic decisions, care planning and genetic counselling. Muscle magnetic resonance imaging (MRI) has emerged as a valuable diagnostic tool by identifying characteristic patterns of muscle involvement. However, the increasing complexity of these patterns complicates their interpretation, limiting their clinical utility. Additionally, multi-study data aggregation introduces heterogeneity challenges. This study presents a novel multi-study harmonization pipeline for muscle MRI and an AI-driven diagnostic tool to assist clinicians in identifying disease-specific muscle involvement patterns.
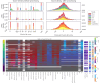
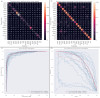
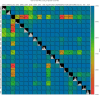
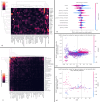
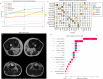
Methods: We developed a preprocessing pipeline to standardize MRI fat content across datasets, minimizing source bias. An ensemble of XGBoost models was trained to classify patients based on intramuscular fat replacement, age at MRI and sex. The SHapley Additive exPlanations (SHAP) framework was adapted to analyse model predictions and identify disease-specific muscle involvement patterns. To address class imbalance, training and evaluation were conducted using class-balanced metrics. The model's performance was compared against four expert clinicians using 14 previously unseen MRI scans.
Results: Using our harmonization approach, we curated a dataset of 2961 MRI samples from genetically confirmed cases of 20 paediatric and adult NMDs. The model achieved a balanced accuracy of 64.8% ± 3.4%, with a weighted top-3 accuracy of 84.7% ± 1.8% and top-5 accuracy of 90.2% ± 2.4%. It also identified key features relevant for differential diagnosis, aiding clinical decision-making. Compared to four expert clinicians, the model obtained the highest top-3 accuracy (75.0% ± 4.8%). The diagnostic tool has been implemented as a free web platform, providing global access to the medical community.
Conclusions: The application of AI in muscle MRI for NMD diagnosis remains underexplored due to data scarcity. This study introduces a framework for dataset harmonization, enabling advanced computational techniques. Our findings demonstrate the potential of AI-based approaches to enhance differential diagnosis by identifying disease-specific muscle involvement patterns. The developed tool surpasses expert performance in diagnostic ranking and is accessible to clinicians worldwide via the Myo-Guide online platform.
Keywords: MRI; artificial intelligence; differential diagnosis; machine learning; neuromuscular diseases.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Mercuri E. and Muntoni F., “Muscular Dystrophies,” Lancet 381, no. 9869 (2013): 845–860. - PubMed
-
- Nuñez‐Peralta C., Alonso‐Pérez J., and Díaz‐Manera J., “The Increasing Role of Muscle MRI to Monitor Changes Over Time in Untreated and Treated Muscle Diseases,” Current Opinion in Neurology 33, no. 5 (2020): 611–620. - PubMed
-
- Dahlqvist J. R., Widholm P., Leinhard O. D., and Vissing J., “MRI in Neuromuscular Diseases: An Emerging Diagnostic Tool and Biomarker for Prognosis and Efficacy,” Annals of Neurology 88, no. 4 (2020): 669–681. - PubMed
-
- Pezeshk P., Alian A., and Chhabra A., “Role of Chemical Shift and Dixon Based Techniques in Musculoskeletal MR Imaging,” European Journal of Radiology 94 (2017): 93–100. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

