Blood biomarkers of vascular dysfunction in small vessel disease progression: Insights from a longitudinal neuroimaging study
- PMID: 40275856
- PMCID: PMC12022501
- DOI: 10.1002/alz.70152
Blood biomarkers of vascular dysfunction in small vessel disease progression: Insights from a longitudinal neuroimaging study
Abstract
Introduction: This study explored the relationship between blood biomarkers of cerebrovascular function and small vessel disease (SVD) neuroimaging markers and cognitive outcomes in highly-phenotyped participants.
Methods: We conducted cross-sectional and 1-year longitudinal analyses on 181 patients with mild ischemic stroke, enriched for SVD features. We examined relationships between a panel of 13 blood biomarkers and magnetic resonance imaging (MRI) markers of SVD (structural lesions, diffusion-weighted imaging [DWI]-positive lesions, blood-brain barrier (BBB) permeability, and cerebrovascular reactivity (CVR), and cognition.
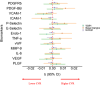
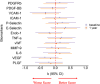
Results: In linear mixed models, vascular endothelial growth factor was significantly associated with incident DWI-positive lesions over 1 year. Intercellular adhesion molecule-1 was linked with lower CVR while platelet-derived growth factor-subunit B and Endothelin-1 were associated with higher CVR. Platelet-Selectin levels were associated with mild cognitive impairment at 1 year.
Discussion: Our results support the role of endothelial and pericyte dysfunction in SVD burden and progression and suggest that specific biomarkers relate to distinct SVD manifestations.
Highlights: Small vessel disease (SVD) lacks specific or predictive biomarker signatures. Vascular endothelial growth factor levels were linked to incident lesions detected over 1 year. Circulating intercellular adhesion molecule-1 related to lower cerebrovascular reactivity. Platelet-selectin levels were associated with mild cognitive impairment longitudinally. These findings could help stratify patients at high-risk of rapid-progression SVD.
Keywords: biomarkers; blood‐brain barrier; cerebrovascular dysfunction; cerebrovascular reactivity; cognitive impairment; endothelial dysfunction; pericyte; small vessel disease; vascular dementia.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Alasdair G. Morgan, Cameron Manning, and Michael S. Stringer were part‐funded by Siemens Healthineers, administered by the University of Edinburgh. The other authors report no conflicts. Author disclosures are available in the Supporting Information.
Figures




References
MeSH terms
Substances
Grants and funding
- Muir Maxwell Research Fund, Edinburgh Imaging
- 2012/17/Edinburgh and Lothians Health Foundation
- R380R/1114/Dunhill Medical Trust
- Scottish Funding Council
- The Row Fogo Centre for Research into Ageing and the Brain: Small Vessel Disease Research
- NHS Lothian Research and Development Office
- The Rowling Clinic
- CAF/18/08/Chief Scientist Office, Scottish Government Health and Social Care Directorate
- AS-CP-18b-001/Alzheimer Society
- The Row Fogo Centre for Research into Aging and the Brain
- RE/18/5/34216/BHF_/British Heart Foundation/United Kingdom
- 16 CVD 05/Fondation Leducq
- UK DRI-4011/UK Dementia Research Institute
- 224912/Z/21/Z/The Wellcome Trust
- 104916/Z/14/Z/The Wellcome Trust
- Stroke Association 'Small Vessel Disease - Spotlight on Symptoms' (SVD-SOS)
- 2021-000007-01EXTF-00234/The Mexican National Council of Science and Technology (CONACYT)
- MR/R502327/The Medical Research Council (MRC) National Productivity Fund
- SAPDF 18∖100026/The Stroke Association
- TSA LECT 2015/04/The Stroke Association
- 16 VAD 07/The Stroke Association
- SA PG 19∖100068/The Stroke Association
LinkOut - more resources
Full Text Sources
Medical

