Characterizing withdrawal from long-acting injectable buprenorphine: An observational case series
- PMID: 40276009
- PMCID: PMC12018199
- DOI: 10.1016/j.dadr.2025.100329
Characterizing withdrawal from long-acting injectable buprenorphine: An observational case series
Abstract
Introduction: Long-acting injectable buprenorphine (LAIB) products are being increasingly used to treat patients with opioid dependence. Limited data is available on the severity or timespan (time to onset, peak, duration) of withdrawal signs and symptoms following discontinuation of treatment.
Methods: Participants aiming to discontinue long-term LAIB treatment commenced the study on the day of their final dose of Buvidal® 64 mg Monthly. Participants were monitored with weekly assessments of withdrawal severity, cravings, general health, and patient experience measures for up to 16 weeks after last dose.
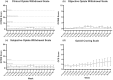
Results: Fifteen participants - those who remained for at least four weeks after the last LAIB dose - were included in the study. There was minimal increase in withdrawal severity over the study period, with an average peak Clinical Opioid Withdrawal Scale score of 4.8 ± 2.7, occurring at a median of 6 weeks (IQR 4-7.5) after the last LAIB dose. Cravings scores were generally low but increased gradually over the 16-week study period. There was no deterioration in physical or mental health scores, and participants reported high levels of satisfaction with the withdrawal experience. Ten participants used rescue medications, predominately in weeks 5 or 6 after the last dose.
Discussion and conclusions: Participants (last dose of Buvidal® 64 mg Monthly) experienced minimal or mild withdrawal signs and symptoms, usually peaking in severity between 5 and 8 weeks after the last dose. These results are encouraging, however clinical trials comparing withdrawal outcomes between LAIB, sublingual buprenorphine (SL BPN) and methadone are required to inform treatment planning.
Keywords: Buprenorphine; Long-acting injectable buprenorphine; Opioid dependence treatment; Opioid withdrawal.
© 2025 South Eastern Sydney Local Health District, Kogarah, Australia.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:The authors declare financial support was provided by Camurus Pty Ltd - manufacturers of Buvidal -for expenses related to the conduct of this trial, however Camurus had no input into the design, collection, analysis, or interpretation of the data presented in this manuscript. There are no other competing interests to report.
Figures
Similar articles
-
Patient Satisfaction and Resource Utilization Following Introduction of Long-Acting Injectable Buprenorphine (LAIB) in Scottish Prisons.Subst Abuse Rehabil. 2025 Apr 15;16:83-93. doi: 10.2147/SAR.S510467. eCollection 2025. Subst Abuse Rehabil. 2025. PMID: 40256212 Free PMC article.
-
Inpatient initiation of long-acting injectable buprenorphine at a community hospital: A retrospective case series.J Addict Dis. 2024 Sep 1:1-7. doi: 10.1080/10550887.2024.2391145. Online ahead of print. J Addict Dis. 2024. PMID: 39219151
-
'Matters-of-concern' associated with discontinuation of long-acting injectable buprenorphine: Findings from a longitudinal qualitative study.Int J Drug Policy. 2024 Jul;129:104470. doi: 10.1016/j.drugpo.2024.104470. Epub 2024 Jun 5. Int J Drug Policy. 2024. PMID: 38843737
-
Prolonged-release buprenorphine formulations: Perspectives for clinical practice.Therapie. 2020 Sep-Oct;75(5):397-406. doi: 10.1016/j.therap.2020.05.007. Epub 2020 May 18. Therapie. 2020. PMID: 32499082 Review.
-
[New slow-release buprenorphine formulations for optimization of opioid substitution].Nervenarzt. 2019 Sep;90(9):932-937. doi: 10.1007/s00115-019-0783-6. Nervenarzt. 2019. PMID: 31399813 Review. German.
References
-
- Albayaty M., Linden M., Olsson H., Johnsson M., Strandgården K., Tiberg F. Pharmacokinetic evaluation of once-weekly and once-monthly buprenorphine subcutaneous injection depots (CAM2038) versus intravenous and sublingual buprenorphine in healthy volunteers under naltrexone blockade: an open-label phase 1 study. Adv. Ther. 2017;34(2):560–575. - PubMed
-
- Atkinson M.J., Sinha A., Hass S.L., Colman S.S., Kumar R.N., Brod M., Rowland C.R. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual. life Outcomes. 2004;2:1–13. - PMC - PubMed
-
- Björnsson M., Acharya C., Strandgården K., Tiberg F. Population pharmacokinetic analysis supports initiation treatment and bridging from sublingual buprenorphine to subcutaneous administration of a buprenorphine depot (CAM2038) in the treatment of opioid use disorder. Clin. Pharmacokinet. 2023;62(10):1427–1443. - PMC - PubMed
-
- Breen C.L., Harris S.J., Lintzeris N., Mattick R.P., Hawken L., Bell J., Ritter A.J., Lenné M., Mendoza E. Cessation of methadone maintenance treatment using buprenorphine: transfer from methadone to buprenorphine and subsequent buprenorphine reductions. Drug Alcohol Depend. 2003;71(1):49–55. - PubMed
-
- Bürkner P.-C. brms: an R package for bayesian multilevel models using stan. J. Stat. Softw. 2017;80(1):1–28.
LinkOut - more resources
Full Text Sources