High-risk features of early relapse in newly-diagnosed multiple myeloma: The impact of cytogenetics and response to initial therapy
- PMID: 40276216
- PMCID: PMC12020018
- DOI: 10.1002/hem3.70127
High-risk features of early relapse in newly-diagnosed multiple myeloma: The impact of cytogenetics and response to initial therapy
Abstract
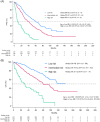
Patients with newly-diagnosed multiple myeloma (MM) who experience early relapse (ER) have dismal overall survival (OS). Their prospective identification, either before or soon after treatment initiation, is paramount to use alternative approaches and prevent ER. In this study, we investigated the frequency and disease characteristics of ER during the first 18 months after treatment initiation (ER18), in a series of 1215 newly-diagnosed MM patients enrolled in four PETHEMA/GEM clinical trials for the transplant-eligible and transplant-ineligible populations. ER18 was observed in 266 of the 1215 patients (22%) and resulted in a median OS of 19 versus 114 months in cases without ER18. When compared to the ISS and the presence of ≥2 high-risk cytogenetic abnormalities, a modified version of the new high-risk definition from the International Myeloma Society (mHR-IMS) showed the most balanced negative and positive predictive values of ER18 (83.5% and 40%, respectively). In addition to the mHR-IMS, an ECOG = 2, ISS 3, and calcium levels ≥ 11 mg/dL were independently associated with ER18. These variables were modeled into a predictive score in which the rates of ER18 were 2%, 24.5%, and 59% in patients with low-, intermediate-, and high-risk score. The risk of ER18 and OS were modulated by the VGPR status at 6-9 months after treatment initiation. In conclusion, we present a risk model that predicts ER18 and can be readily applied in clinical trials and routine practice to identify treatment strategies empowered to prevent ER18 and improve survival outcomes of newly-diagnosed patients with functional high-risk MM.
© 2025 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
A. M. declares no conflicts of interest. P. R.‐O. declares honoraria derived from consulting or advisory board roles with Celgene‐BMS, Janssen, Roche, AbbVie, Pfizer, GSK, Sanofi, and H3Biomedicine; steering committee membership with Celgene‐BMS, Regeneron, and Janssen; speakers' bureau for Janssen, Celgene‐BMS, GSK, Sanofi, and AbbVie; and travel grants from Pfizer. J. S. M. declares honoraria for lectures and particiption on advisory boards from AbbVie, Amgen, BMS, Celgene, F. Hoffman‐La Roche, GSK, HaemaLogiX, Jansen‐Cilag, Karyopharm, Merck, Novartis, Pfizer, Regeneron, Sanofi‐Aventis, Takeda, and SecuraBio. B. P. declares honoraria for lectures from and membership on advisory boards with Adaptive, Amgen, Bristol‐Myers Squibb‐Celgene, Gilead, GSK, Janssen, Oncopeptides, Roche, Sanofi, Takeda, and The Binding Site; unrestricted grants from Bristol‐Myers Squibb‐Celgene, EngMab, GSK, Roche, Sanofi, and Takeda; and consultancy for Bristol‐Myers Squibb‐Celgene, Janssen, and Sanofi. V. C. received honoraria for lectures, consulting, and advisory boards from Johnson & Johnson, BMS, Sanofi, Amgen, Glaxo, Pfizer, BioGene, Menarini; travel grants from Johnson & Johnson, BMS, Amgen, BioGene; speakers' bureau participation for BMS; and financing of scientific research from Janssen. N. C. G. has received honoraria from Janssen, Amgen, and Sanofi. L. R. reports honoraria from Janssen, Celgene, Amgen, and Takeda. A. O. declares consultant activities with Amgen, Celgene, BMS, GSK, Janssen, and Sanofi. J. M.‐L. declares honoraria from consulting activities from Astellas Pharma, BMS, F. Hoffman‐La Roche, Janssen, Novartis, and Sanofi; and a research grant from BMS. M. V. M. declares honoraria derived from lectures and participation in advisory boards with Johnson & Johnson, Celgene‐BMS, Amgen, Takeda, Sanofi, GSK, Pfizer, Kite, Oncopeptides, Stemline‐Menarini, Regeneron, and AbbVie. J. d. l. R. declares honoraria derived from lectures and participation in advisory boards with Johnson & Johnson, Celgene‐BMS, Takeda, Sanofi, GSK, Pfizer, and Stemline‐Menarini. E. M. O. declares honoraria/consulting fees from AbbVie, Amgen, AstraZeneca, BMS, GSK, Janssen, Karyopharm, Menarini, Oncopeptides, Pfizer, Regeneron, Sanofi, and Takeda. M. G. reports consulting fees, honoraria, and participation in advisory boards for J&J, Amgen, Sanofi, Pfizer, and Menarini Stemline. F. d. A. declares honoraria derived from lectures and participation in advisory boards with Johnson & Johnson, Celgene‐BMS, Amgen, GSK, Sanofi, and Takeda. J. B. declares honoraria for lectures from Janssen, Celgene/BMS, Amgen, and Sanofi. J. M. A. declares honoraria for lectures and advisory boards from Johnson & Johnson, Sanofi, GSK, Amgen, Roche, Bristol‐Myers Squibb, and BindingSite. A. A. declares honoraria derived from lectures and participation in advisory boards with Amgen, Johnson & Johnson, Celgene‐BMS, Sanofi, Pfizer, Oncopeptides, GSK, and Regeneron. J. J. L. declares consulting or advisory roles with Celgene, Amgen, Janssen‐Cilag, and Sanofi; and travel, accommodations, and expenses were provided by Celgene and Pfizer. The remaining authors declare no competing interests relative to this work.
Figures

References
-
- Moreau P, Hulin C, Perrot A, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab and followed by daratumumab maintenance or observation in transplant‐eligible newly diagnosed multiple myeloma: long‐term follow‐up of the CASSIOPEIA randomised controlled phase 3 trial. Lancet Oncol. 2024;25(8):1003‐1014. - PubMed
-
- Moreau P, Cavo M, Sonneveld P, et al. Combination of International Scoring System 3, high lactate dehydrogenase, and t(4;14) and/or del(17p) identifies patients with multiple myeloma (MM) treated with front‐line autologous stem‐cell transplantation at high risk of early MM progression‐related death. J Clin Oncol. 2014;32(20):2173‐2180. - PMC - PubMed
-
- Sonneveld P, Dimopoulos MA, Boccadoro M, et al. Daratumumab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2024;390(4):301‐313. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
