Desmoplastic tumor priming using clinical-stage corticosteroid liposomes
- PMID: 40276304
- PMCID: PMC12014906
- DOI: 10.1016/j.celbio.2025.100051
Desmoplastic tumor priming using clinical-stage corticosteroid liposomes
Abstract
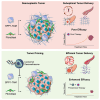
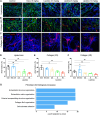
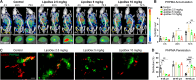
Inflammation is a hallmark of cancer. It contributes to a heterogeneous, hyperpermeable, and poorly perfused tumor vasculature, as well as to a dense and disorganized extracellular matrix, which together negatively affect drug delivery. Reasoning that glucocorticoids have pleiotropic effects, we use clinical-stage dexamethasone liposomes (LipoDex) to prime the tumor microenvironment for improved drug delivery and enhanced treatment efficacy. We show that LipoDex priming improves tumor vascular function and reduces extracellular matrix deposition. Single-cell sequencing corroborates LipoDex-mediated inhibition of pro-inflammatory, pro-angiogenic, and pro-fibrogenic gene expression in mononuclear cells, tumor-associated macrophages, and cancer-associated fibroblasts. Multimodal optical imaging illustrates that LipoDex pre-treatment increases the tumor accumulation and intratumoral distribution of subsequently administered polymeric and liposomal drug delivery systems. Using Doxil as a prototypic nanodrug, we finally show that LipoDex priming promotes antitumor treatment efficacy. Altogether, our findings demonstrate that desmoplastic tumors can be primed for improved drug targeting and therapy using clinical-stage glucocorticoid liposomes.
Keywords: cancer-associated fibroblasts; corticosteroids; drug targeting; liposomes; nanomedicine; tumor microenvironment; tumor priming; tumor-associated macrophages.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Nguyen L.N.M., Ngo W., Lin Z.P., Sindhwani S., MacMillan P., Mladjenovic S.M., Chan W.C.W. The mechanisms of nanoparticle delivery to solid tumours. Nat Rev Bioeng. 2024;2:201–213. doi: 10.1038/s44222-024-00154-9. - DOI
LinkOut - more resources
Full Text Sources
