Establishment of a pseudovirus neutralization assay for TGEV
- PMID: 40276514
- PMCID: PMC12018367
- DOI: 10.3389/fimmu.2025.1558604
Establishment of a pseudovirus neutralization assay for TGEV
Abstract
Introduction: Transmissible Gastroenteritis Virus (TGEV) is a major pathogen causing swine enteric diseases, necessitating effective control strategies. Vaccination plays a key role, but assessing vaccine efficacy remains challenging due to variations in immune response and existing detection limitations. Current antibody detection methods, such as neutralization assays and ELISA, are often subjective, labor-intensive, and time-consuming, highlighting the need for a more efficient evaluation approach.
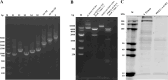
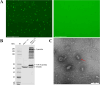
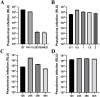
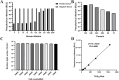
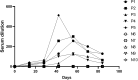
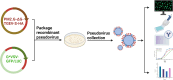
Methods and results: The TGEV S gene was amplified and inserted into the eukaryotic vector PM2.G-ΔG-HA to construct the recombinant plasmid PM2.G-ΔG-TGEV-S-HA. Transfecting ST cells with this plasmid, followed by infection with G*VSV-GFP/LUC, successfully produced TGEV P0 pseudoviruses. Western blot and electron microscopy confirmed the presence of TGEV S and VSV N proteins and the distinct pseudovirus morphology. Optimization determined that 0.5 μg/well of plasmid, 24 h transfection, and 24 h post-infection harvest yielded a viral titer of 106-107 TCID50/mL. The pseudoviruses exhibited strong ST cell tropism and were effectively neutralized by TGEV-positive sera. A pseudovirus-based neutralization test (pNT) was established, showing 100% sensitivity, 96.6% specificity, no cross-reactivity with PEDV, PPV, PDCoV, or PRoV, and a 94% concordance with the live virus neutralization test. The method effectively tracked antibody level changes post-TGEV vaccination.
Discussion: This study successfully developed a novel pseudovirus-based detection method, overcoming traditional assay limitations. The pNT method provides a scalable, efficient, and reliable tool for TGEV antibody evaluation, with broad potential applications in pathogen detection and vaccine assessment.
Keywords: ST cells; TGEV; neutralizing antibody; pseudovirus; pseudovirus neutralization test.
Copyright © 2025 Wang, Chen, Xue, Sun, An, Wang, Chen, Yu, Xia and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Evaluation on the efficacy and immunogenicity of recombinant DNA plasmids expressing spike genes from porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus.PLoS One. 2013;8(3):e57468. doi: 10.1371/journal.pone.0057468. Epub 2013 Mar 19. PLoS One. 2013. PMID: 23526943 Free PMC article.
-
Antigenic relationships among porcine epidemic diarrhea virus and transmissible gastroenteritis virus strains.J Virol. 2015 Mar;89(6):3332-42. doi: 10.1128/JVI.03196-14. Epub 2015 Jan 14. J Virol. 2015. PMID: 25589635 Free PMC article.
-
Evaluation of the baculovirus-expressed S glycoprotein of transmissible gastroenteritis virus (TGEV) as antigen in a competition ELISA to differentiate porcine respiratory coronavirus from TGEV antibodies in pigs.J Vet Diagn Invest. 1999 May;11(3):205-14. doi: 10.1177/104063879901100301. J Vet Diagn Invest. 1999. PMID: 10353350
-
An overview of immunological and genetic methods for detecting swine coronaviruses, transmissible gastroenteritis virus, and porcine respiratory coronavirus in tissues.Adv Exp Med Biol. 1997;412:37-46. doi: 10.1007/978-1-4899-1828-4_4. Adv Exp Med Biol. 1997. PMID: 9191988 Review.
-
Immunity to transmissible gastroenteritis virus and porcine respiratory coronavirus infections in swine.Vet Immunol Immunopathol. 1994 Oct;43(1-3):89-97. doi: 10.1016/0165-2427(94)90124-4. Vet Immunol Immunopathol. 1994. PMID: 7856068 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

