Endothelial cell supplementation promotes xenograft revascularization during short-term ovarian tissue transplantation
- PMID: 40276538
- PMCID: PMC12020896
- DOI: 10.1016/j.bioactmat.2025.03.021
Endothelial cell supplementation promotes xenograft revascularization during short-term ovarian tissue transplantation
Abstract
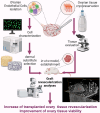
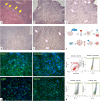
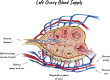
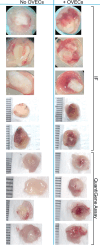
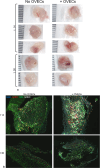
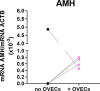
The ischemic/hypoxic window after Ovarian Tissue Transplantation (OTT) can be responsible for the loss of more than 60 % of follicles. The implantation of the tissue supplemented with endothelial cells (ECs) inside dermal substitutes represents a promising strategy for improving graft revascularization. Ovarian biopsies were partly cryopreserved and partly digested to isolate ovarian ECs (OVECs). Four dermal substitutes (Integra®, made of bovine collagen enriched with chondroitin 6-sulfate; PELNAC®, composed of porcine collagen; Myriad Matrix®, derived from decellularized ovine forestomach; and NovoSorb® BMT, a foam of polyurethane) were compared for their angiogenic bioactive properties. OVECs cultured onto the scaffolds upregulated the expression of angiogenic factors, supporting their use in boosting revascularization. Adhesion and proliferation assays suggested that the most suitable scaffold was the bovine collagen one, which was chosen for further in vivo experiments. Cryopreserved tissue was transplanted onto the 3D scaffold in immunodeficient mice with or without cell supplementation, and after 14 days, it was analyzed by immunofluorescence (IF) and X-ray phase contrast microtomography. The revascularization area of OVECs-supplemented tissue was doubled (7.14 %) compared to the scaffold transplanted alone (3.67 %). Furthermore, tissue viability, evaluated by nuclear counting, was significantly higher (mean of 169.6 nuclei/field) in the tissue grafted with OVECs than in the tissue grafted alone (mean of 87.2 nuclei/field). Overall, our findings suggest that the OVECs-supplementation shortens the ischemic interval and may significantly improve fertility preservation procedures.
Keywords: Endothelial cells; OTT mouse model; Ovarian tissue transplantation; Revascularization.
© 2025 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures











References
-
- Dolmans M.M., Donnez J., Cacciottola L. Fertility preservation: the challenge of freezing and transplanting ovarian tissue. Trends Mol. Med. 2020;27:777–791. - PubMed
-
- Cacciottola L., Donnez J., Dolmans M.M. Ovarian tissue and oocyte cryopreservation prior to iatrogenic premature ovarian insufficiency. Best Pract. Res. Clin. Obstet. Gynaecol. 2022;81:119–133. - PubMed
-
- Mishra S.R., Chung H.F., Waller M., Mishra G.D. Duration of estrogen exposure during reproductive years, age at menarche and age at menopause, and risk of cardiovascular disease events, all-cause and cardiovascular mortality: a systematic review and meta-analysis. BJOG. 2021;128:809–821. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

