Exomeres and supermeres: Current advances and perspectives
- PMID: 40276541
- PMCID: PMC12020890
- DOI: 10.1016/j.bioactmat.2025.04.012
Exomeres and supermeres: Current advances and perspectives
Abstract
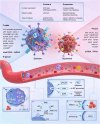
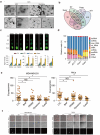
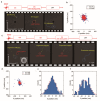
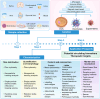
Recent studies have revealed a great diversity and complexity in extracellular vesicles and particles (EVPs). The developments in techniques and the growing awareness of the particle heterogeneity have spurred active research on new particle subsets. Latest discoveries highlighted unique features and roles of non-vesicular extracellular nanoparticles (NVEPs) as promising biomarkers and targets for diseases. These nanoparticles are distinct from extracellular vesicles (EVs) in terms of their smaller particle sizes and lack of a bilayer membrane structure and they are enriched with diverse bioactive molecules particularly proteins and RNAs, which are widely reported to be delivered and packaged in exosomes. This review is focused on the two recently identified membraneless NVEPs, exomeres and supermeres, to provide an overview of their biogenesis and contents, particularly those bioactive substances linked to their bio-properties. This review also explains the concepts and characteristics of these nanoparticles, to compare them with other EVPs, especially EVs, as well as to discuss their isolation and identification methods, research interests, potential clinical applications and open questions.
Keywords: Disease diagnosis and treatment; Exomeres; Nanoparticles; Separation and extraction; Supermeres.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

