Two decades of experience of the Fabry Outcome Survey provides further confirmation of the long-term effectiveness of agalsidase alfa enzyme replacement therapy
- PMID: 40276560
- PMCID: PMC12018052
- DOI: 10.1016/j.ymgmr.2025.101215
Two decades of experience of the Fabry Outcome Survey provides further confirmation of the long-term effectiveness of agalsidase alfa enzyme replacement therapy
Abstract
Background: Analyses of up to 20 years of data from the Fabry Outcome Survey (FOS) assessed the long-term effectiveness of agalsidase alfa enzyme replacement therapy.
Methods: The impact of agalsidase alfa treatment on renal, cardiac, morbidity, and mortality outcomes in FOS was compared with untreated external Fabry disease (FD) cohorts.
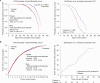
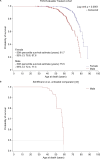
Results: A total of 2171 FOS patients (1014 men, 919 women, 163 boys, 75 girls) received agalsidase alfa (median [range] duration of treatment: 5.38 [0.0-20.8] years). Annual rates of decline in estimated glomerular filtration rate improved in treated patients versus untreated external cohorts regardless of sex or baseline urinary protein levels. Annual left ventricular mass index rates were stable in treated patients regardless of sex or baseline left ventricular hypertrophy status, and better than in untreated external cohorts. The mean age at which 50 % of patients had their first composite morbidity event was later in the agalsidase-alfa-treated population than in the untreated external cohort (51.7 vs 41 years [males]; 60.8 vs 53 years [females]). After 24 months of treatment, the probability of a composite morbidity event was ∼34 % in treated patients and ∼ 45 % in untreated patients. Treated patients were older at death than untreated patients (mean [range]: 61.7 [26.2-87.6] vs 50.3 [34.5-70.1] years). The mean age at which 50 % of male patients were still alive was higher in treated patients than in untreated external cohorts (75.5 vs 60.0 years).
Conclusions: Long-term treatment with agalsidase alfa may provide renal, cardiac, and overall survival protection in FD.
Keywords: Agalsidase alfa; Clinical outcomes; Fabry disease; Long-term data; Registry data.
© 2025 The Authors.
Conflict of interest statement
UR reports honoraria for speaking and/or advisory boards from Amicus Therapeutics, Sanofi Genzyme, and Takeda, and research grants from Amicus Therapeutics, Intrabio, and Takeda. She is a member of the FOS Steering Committee. GP-M reports honoraria from Alexion, Amicus Therapeutics, BioMarin, Chiesi, Kyowa-Kirin, Lucane, Sanofi, and Takeda, and unrestricted grants from Sanofi and Takeda to the Vall d'Hebron 10.13039/100005930Research Foundation for funding research on rare diseases. He is a member of the FOS Steering Committee. CK reports honoraria for speaking and/or advisory boards from Amicus Therapeutics, BioMarin, Gore, and Takeda. He is a member of the FOS Steering Committee. KN reports honoraria from Amicus Therapeutics, Sanofi Genzyme, and Takeda. She is a member of the FOS Steering Committee. D-MN reports honoraria and speaker fees from Sanofi Genzyme and Takeda, and research grants from BioMarin, Sanofi Genzyme, and Takeda. He is a member of the FOS Steering Committee. RR reports honoraria, speaker fees, and consulting fees from Amicus Therapeutics, CSL Behring, Gador, Novartis, Sanofi Genzyme, and Takeda. He is a member of the FOS Steering Committee. MLW reports grants, personal fees, and travel support from 10.13039/100006396Alexion, Alnylam, 10.13039/100015362Amicus Therapeutics, Chiesi, Idorsia, Moderna, Protalix, 10.13039/100013995Sanofi Genzyme, and Takeda. He is a member of the FOS Steering Committee. CA is a former employee of Takeda Pharmaceuticals International AG and a current employee of Medison Pharma, Switzerland. JB is an employee of Takeda Pharmaceuticals International AG and is a stockholder of Takeda Pharmaceuticals Company Limited. DJ is an employee of Takeda Pharmaceuticals International AG and is a stockholder of Takeda Pharmaceuticals Company Limited. JS is a former employee of Takeda Pharmaceuticals International AG and a current employee of Kalvista Pharmaceuticals, Switzerland. DAH reports honoraria from Amicus Therapeutics, Chiesi, Freeline Therapeutics, Idorsia, Protalix, Sanofi Genzyme, and Takeda. She is a member of the FOS Steering Committee. RG reports honoraria, consulting fees, speaker fees, research funding, and/or travel reimbursement from Alexion, Allievex, Alnylam, Amicus Therapeutics, Astellas, Azafaros, BioMarin, Chiesi, JCR, Lysogene, Novartis, Paradigm, PassageBio, Pfizer, Praxis, PTC, Regenxbio, Sanofi, Takeda, and Ultragenyx. He is a member and the current chair of the FOS Steering Committee.
Figures


References
-
- Sweeley C.C., Klionsky B. Fabry’s disease: classification as a sphingolipidosis and partial characterization of a novel glycolipid. J. Biol. Chem. 1963;238:3148–3150. - PubMed
-
- Brady R.O., Gal A.E., Bradley R.M., Martensson E., Warshaw A.L., Laster L. Enzymatic defect in Fabry's disease. Ceramidetrihexosidase deficiency. N. Engl. J. Med. 1967;276:1163–1167. - PubMed
-
- Kint J.A. Fabry's disease: alpha-galactosidase deficiency. Science. 1970;167:1268–1269. - PubMed
-
- Zivna M., Dostalova G., Baresova V., Musalkova D., Svojsova K., Meiseles D., Kinstlinger S., Kuchar L., Asfaw B., Poupetova H., Vlaskova H., Kmochova T., Vyletal P., Hartmannova H., Hodanova K., Stranecky V., Steiner-Mrazova L., Hnizda A., Zivny J., Radina M., Votruba M., Sovova J., Treslova H., Stolnaja L., Rekova P., Roblova L., Honsova E., Rychlik I., Dvela-Levitt M., Bleyer A.J., Linhart A., Sikora J., Kmoch S. Misprocessing of alpha-galactosidase A, endoplasmic reticulum stress, and the unfolded protein response. J. Am. Soc. Nephrol. 2024;36:628–644. doi: 10.1681/ASN.0000000535. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

