Circulating tumor DNA monitoring in advanced mutated melanoma (LIQUID-MEL)
- PMID: 40276578
- PMCID: PMC12019447
- DOI: 10.1016/j.jlb.2025.100295
Circulating tumor DNA monitoring in advanced mutated melanoma (LIQUID-MEL)
Abstract
Introduction: Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of metastatic melanoma, but a percentage of patients did not show benefit. Circulating tumor DNA (ctDNA) has emerged as a potential non-invasive tool for monitoring disease evolution and treatment response. The present study aimed to evaluate the clinical utility of ctDNA dynamics in patients with metastatic melanoma receiving ICIs, while exploring its role in the oncological course.
Materials and methods: The LIQUID-MEL study is a prospective, single-centre pilot study including patients with BRAF/NRAS-mutant metastatic melanoma. ctDNA was quantified using digital droplet PCR (ddPCR) at four different time points. Uni- and multivariable Cox regression models were used to assess the correlation between shedding and progression-free survival (PFS), and overall survival (OS).
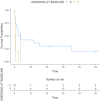
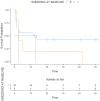
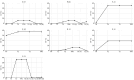
Results: Overall, 23 patients were included. At baseline, ctDNA was detectable in 5/23 (21.7 %) cases. Baseline ctDNA shedding was associated with shorter PFS (3.88 months vs. 0.69 months, p=0.012). A strong numerical trend was observed also in OS (12.66 months vs. 2.53 months, p=0.287). Shedding at baseline did not demonstrate independent prognostic or predictive value in the uni- and multivariable analysis. The longitudinal analysis revealed intriguing patterns of ctDNA shedding in individual patients.
Conclusion: ctDNA detectability and its dynamic changes during treatment may have potential clinical utility in patients with metastatic melanoma, offering a valuable non-invasive tool for monitoring disease and treatment response. The small sample size limited the statistical power of the analysis. Further studies with larger cohorts are needed to validate its role in routine clinical practice.
Keywords: Immune checkpoint inhibitors; Liquid biopsy; Melanoma; Monitoring; Prognosis; ctDNA.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Dynamic bTMB combined with residual ctDNA improves survival prediction in locally advanced NSCLC patients with chemoradiotherapy and consolidation immunotherapy.J Natl Cancer Cent. 2024 Apr 10;4(2):177-187. doi: 10.1016/j.jncc.2024.01.008. eCollection 2024 Jun. J Natl Cancer Cent. 2024. PMID: 39282582 Free PMC article.
-
Application of Circulating Cell-Free Tumor DNA Profiles for Therapeutic Monitoring and Outcome Prediction in Genetically Heterogeneous Metastatic Melanoma.JCO Precis Oncol. 2019 Feb 15;3:PO.18.00229. doi: 10.1200/PO.18.00229. eCollection 2020. JCO Precis Oncol. 2019. PMID: 32914028 Free PMC article.
-
Circulating tumour DNA in patients with advanced melanoma treated with dabrafenib or dabrafenib plus trametinib: a clinical validation study.Lancet Oncol. 2021 Mar;22(3):370-380. doi: 10.1016/S1470-2045(20)30726-9. Epub 2021 Feb 12. Lancet Oncol. 2021. PMID: 33587894 Free PMC article. Clinical Trial.
-
The prognostic value of circulating tumor DNA in patients with melanoma: A systematic review and meta-analysis.Transl Oncol. 2021 Jun;14(6):101072. doi: 10.1016/j.tranon.2021.101072. Epub 2021 Mar 18. Transl Oncol. 2021. PMID: 33744725 Free PMC article. Review.
-
Changes in circulating tumor DNA and outcomes in solid tumors treated with immune checkpoint inhibitors: a systematic review.J Immunother Cancer. 2023 Feb;11(2):e005854. doi: 10.1136/jitc-2022-005854. J Immunother Cancer. 2023. PMID: 36792122 Free PMC article.
References
-
- Robert C., Long G.V., Brady B., et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. - PubMed
-
- Schachter J., Ribas A., Long G.V., et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390(10105):1853–1862. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

