Pilot Findings From the First Legalized Mobile Retail Pharmacy Clinic in the United States for Infectious Disease Treatment and Prevention Tailored to Reach People Who Use Drugs
- PMID: 40276721
- PMCID: PMC12019630
- DOI: 10.1093/ofid/ofaf200
Pilot Findings From the First Legalized Mobile Retail Pharmacy Clinic in the United States for Infectious Disease Treatment and Prevention Tailored to Reach People Who Use Drugs
Abstract
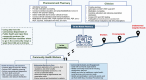
Background: Mobile retail pharmacies were legalized in Connecticut in 2023 to provide primary care, human immunodeficiency virus (HIV) and hepatitis C virus (HCV) testing, preexposure prophylaxis (PrEP), immediate HIV antiretroviral therapy (ART), and medications for substance use disorders directly to people who use drugs (PWUD).
Methods: InMOTION mobile pharmacy and clinic (MPC) pilot findings describe services provided by pharmacists, clinicians, and community health workers.
Results: From 13 December 2023 through 5 November 2024, the MPC engaged with 414 participants, of whom 43% were female, 26% Black/African American, 32% uninsured, and 37% unhoused or unstably housed. Fifty-one had a previous diagnosis of an opioid use disorder (OUD), 163 accepted screening, 1 received a new diagnosis of moderate to severe OUD, and 37 received medication for OUD. Nine participants requested sexually transmitted infection testing; 3 people had positive results, all were prescribed treatment, and 1 received doxycycline postexposure prophylaxis. Four people had existing HIV diagnoses; 166 accepted rapid point-of-care (POC) testing, resulting in 1 positive test; all received ART (2 oral, 3 injectable); 9 who tested HIV negative accepted PrEP, and 1 accepted the injectable formulation. Twenty-two had known HCV, 157 accepted rapid POC HCV testing, 9 tested positive for HCV antibodies, and 11 underwent HCV viral load (VL) testing; 1 self-cleared, and 8 of 10 with detectable HCV VL received direct-acting antivirals from the MPC. Six were treated for xylazine-related wounds.
Conclusions: Health services delivered through an MPC demonstrate the potential to address healthcare gaps for PWUD and warrant exploration and expansion.
Keywords: HCV; HIV; PrEP; mobile pharmacy; substance use disorder.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures
Similar articles
-
Treatment of Hepatitis C virus among people who inject drugs at a syringe service program during the COVID-19 response: The potential role of telehealth, medications for opioid use disorder and minimal demands on patients.Int J Drug Policy. 2022 Mar;101:103570. doi: 10.1016/j.drugpo.2021.103570. Epub 2021 Dec 20. Int J Drug Policy. 2022. PMID: 34954493 Free PMC article.
-
Sexually Transmitted Infection (STI) Incidence, STI Screening, and Human Immunodeficiency Virus Preexposure Prophylaxis Uptake in United States Veterans With Opioid Use Disorder in Long Island, New York.Open Forum Infect Dis. 2024 Jul 22;11(8):ofae429. doi: 10.1093/ofid/ofae429. eCollection 2024 Aug. Open Forum Infect Dis. 2024. PMID: 39086462 Free PMC article.
-
Management of hepatitis C in decentralised versus centralised drug substitution programmes and minimally invasive point-of-care tests to close gaps in the HCV cascade.Swiss Med Wkly. 2017 Nov 20;147:w14544. doi: 10.4414/smw.2017.14544. eCollection 2017. Swiss Med Wkly. 2017. PMID: 29185250
-
Study protocol of a randomized controlled trial comparing two linkage models for HIV prevention and treatment in justice-involved persons.BMC Infect Dis. 2022 Apr 15;22(1):380. doi: 10.1186/s12879-022-07354-x. BMC Infect Dis. 2022. PMID: 35428213 Free PMC article.
-
Attitudes toward pharmacy-based HCV/HIV testing among people who use drugs in rural Kentucky.J Rural Health. 2022 Jan;38(1):93-99. doi: 10.1111/jrh.12564. Epub 2021 Mar 5. J Rural Health. 2022. PMID: 33666274 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

