Birch pollen allergen-induced dsDNA release activates cGAS-STING signaling and type 2 immune response in mice
- PMID: 40276777
- PMCID: PMC12018559
- DOI: 10.1016/j.isci.2025.112324
Birch pollen allergen-induced dsDNA release activates cGAS-STING signaling and type 2 immune response in mice
Abstract
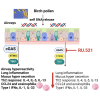
Detecting cytoplasmic or extracellular DNA from host or pathogen origin by DNA sensor cyclic GMP-AMP synthase (cGAS) and stimulator of interferon genes (STING) triggers immune responses with secretion of type I interferons and inflammatory cytokines. However, STING agonists function as type-2 adjuvant promoting allergic asthma. Here, we asked how cGAS/STING signaling pathway influences allergen-induced type-2 immune responses in models of allergic airway diseases induced by birch pollen extract, house dust mite, or ovalbumin plus Alum. We report increased extracellular dsDNA in the airways, together with cGAS and STING gene expression, following allergen challenge in these models, correlating dsDNA and type-2 cytokine IL-4, IL-5, and IL-13 release. Allergen-induced type-2 immune responses were reduced in cGAS- or STING-deficient mice. Further, blocking cGAS function with the specific inhibitor RU.521 protected mice from birch pollen allergen-induced airway inflammation and type-2 immune responses. Thus, DNA sensing by cGAS contributes to type-2 immune responses and may represent a therapeutic target for allergic lung inflammation.
Keywords: Immune response; Immunology; Molecular biology.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Toussaint M., Jackson D.J., Swieboda D., Guedán A., Tsourouktsoglou T.D., Ching Y.M., Radermecker C., Makrinioti H., Aniscenko J., Bartlett N.W., et al. Host DNA released by NETosis promotes rhinovirus-induced type-2 allergic asthma exacerbation. Nat. Med. 2017;23:681–691. doi: 10.1038/nm.4332. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

